AmericanChemicalSociety.com
Reports: UR6 50147-UR6: Understanding the Role of Metal Ions in Mass Spectrometric Ionization Processes: Experimental and Computational Studies
Farooq A. Khan, PhD, University of West Georgia
The proposal was based on computational and experimental studies of binding of metal ions to synthetic organic compounds that serve as matrices in MALDI experiments. While the author has a working knowledge of Gaussian 03 at the time of writing this proposal, an excellent opportunity for enhancing his knowledge of this software was afforded by the week-long workshop and mini conference organized by Gaussian, Inc., in June, 2010. With partial funding from ACS-PRF and support from an intramural technology fee grant, the site license for the upgraded version (Gaussian 09) was purchased, installed on several computers, and used for faculty-directed undergraduate research.
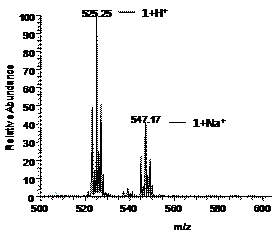
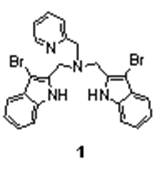
With permission from the Program Officer, the mass spectral studies were extended to include electrospray ionization mass spectrometry (ESI-MS). One of the compounds studied for both cation and anion binding properties was the ligand 1, (Figure 1) synthesized by Dr. Megumi Fujita, a past recipient of the ACS-PRF grant (45505-GB3). Interestingly, the ligand exhibited formation of adducts with both alkali metal ions and halide ions in ESI-MS experiments. For the cations, the intensities of the adducts (obtained in methanol with a five-fold excess of the alkali metal ion) varied as Li+~Na+>K+. For halide ions, the intensities of the adducts (obtained in methanol with a five-fold excess of the halide ion) varied as Cl->Br->I-. Representative mass spectra are shown below in Figures 2 and 3.
Figure 1. The Ligand 1. Figure 2. Positive-ion
ESI mass spectrum of 1:5 mole ratio of 1:NaPF6 in methanol. Figure 3. Negative-ion
ESI mass spectrum of 1:5 mole ratio of 1:Cl-
in methanol.
Quantitative studies of anion
binding were conducted via NMR titrations.
The association constants Ka (M-1) of the
formation of 1•X- adducts from 1 and Bu4NX
(X = F, Cl, Br, I) based on NMR titrations of in CD3CN
were fond to be 1042±21, 157±9 and 12±1 M-1, respectively, for Cl-, Br- and I-, while
that for F- is too small to be measured.
Interestingly, the ligand 1 also
shows ditopic behavior, i.e., simultaneous binding
with cations and anions. This is demonstrated in NMR titrations, and
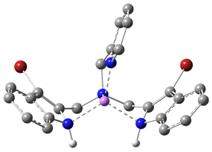
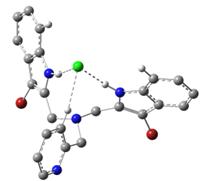
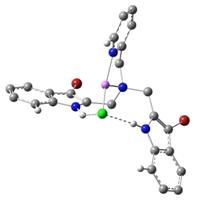
very conclusively via density functional theory (DFT) calculations. In Figure 4, the binding of a Li+
ion only, a chloride ion only, and both lithium and chloride ions to the ligand 1 are
shown in DFT-optimized structures, obtained using the program Gaussian 09.
1•Li+
1•Cl-
1•Cl-•Li+
Figure 4. DFT-optimized
structures: Color scheme: Gray = C, white = H, blue = N, scarlet = Br, green = Cl and pink = Li. Some H atoms are omitted for clarity. The work described above, carried
out in collaboration with undergraduate students, has been accepted for
publication in Tetrahedron Letters.
Work in progress includes the
theoretical (DFT) and experimental (NMR and ESI-MS) investigation of the
interaction of TBA halides in solution.
In particular, the formation of [TBA2X]+
and [TBAX2]- complexes is currently being explored. In addition, the formation of adducts of a
variety of MALDI matrices and alkali and alkaline earth metal ions are also
being studied both my DFT and ESI-MS techniques.
Copyright © American Chemical Society