AmericanChemicalSociety.com
Reports: G7 47744-G7: Structure Engineering of Side Chain Perylene Tetracarboxylic Diimide Diblock Copolymers
Shi Jin, City University of New York (Staten Island)
1. We have developed two synthetic approaches toward unsymmetric PDIs
In approach A, the unsymmetrically substituted PDI 1s was prepared in a high yield and under a mild condition as shown in scheme 1. Variations of 1 were conveniently transformed to an array of unsymmetrically substituted PDIs including amphiphilic PDIs and monomers of PDI side-chain polymers.
In approach B,
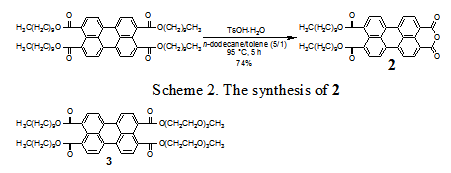
the versatile intermediate 2 was
prepared in a good yield as presented in Scheme 2. 2 was successfully converted into a series of unsymmetrically
substituted PDIs. Moreover, the first
unsymmetrically substituted perylene tetracarboxylic ester 3 was successfully prepared from 2. Perylene tetracarboxylic
esters are also good organic electron acceptors, however their applications
other than simple symmetrically tetrasubstituted compounds are very rare,
probably due to the difficulty in accessing unsymmetrically substituted
compounds. The method we developed may
catalyze the further development of perylene tetracarboxylic tetraesters, particularly their incorporation in
complicated molecular and supramolecular systems and polymers.
2. We have performed unsymmetric tuning of PDIs
Equipped with the enabling
chemistry, unsymmetric PDI p-stack tuning has been performed. This type of tuning has been done at both
sub-nanometer and nanometer levels.
At sub-nanometer level, the
tunability of PDI p-stacks
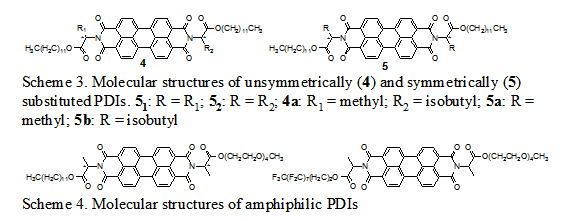
was further enhanced by the synthesis of unsymmetrically substituted 4s. It is reasonable to expect the
phase behavior of 4 to be between
that of two corresponding symmetrically substituted 51 and 52. On one hand, the expected behaviors were
indeed observed for most variations of 4. On the other hand, a few exceptions
occurred. For instance, both 5a and 5b are optically anisotropic liquid crystalline materials at room
temperature. However, at room
temperature compound 4a
self-assembles into a novel optically isotropic phase with p-stacked
PDI moieties. An interesting electric
switching phenomenon was observed when the electric field applied to 4a is greater than a threshold value.
Intramolecular
microphase segregation was utilized to control the
organization of PDI p-stacks
at nanometer level. In addition to the
microphase segregation between the rigid PDI core and flexible side-chains,
incompatible side-chains were introduced to PDIs. We have synthesized a number of amphiphilic
PDIs with two examples given in Scheme 4.
Structural analysis of these compounds indicated that alkyl/tetraethylene glycol and alkyl/perfluoalkyl
microphase segregation indeed occur and lead to the
formation of novel phase structures.
3. p-Stack tuning in molecular
glass
In many applications of organic
conjugated materials, it is important to be able to obtain homogeneous,
transparent thin films. In this regard,
amorphous glassy materials exhibit isotropic properties and it is their
inherent ability to form homogenous and transparent films. Although polymers are the most common sources
of amorphous glassy materials, amorphous glassy materials derived from small
molecules, i.e. molecular glasses, offer the advantage of well-defined
composition, high purity and good processability. However, molecular glasses are usually
thermodynamically unstable. Thus they tend to order quickly unless the ordering
process is kinetically depressed by a glass transition temperature
significantly higher than the application temperature. Despite their technique usefulness, there are
few reports on PDI molecular glasses with high Tgs. By introducing rigid side groups, we have
successfully synthesized a series of amorphous glassy PDIs. They have been thoroughly characterized by
polarizing optical microscopy, X-ray diffraction, UV-Visible spectroscopy,
differential scanning calorimetry, atomic force
microscopy and charge carrier mobility measurements. All of them exhibit a
glass transition temperature higher than 100 °C. The electron mobility is as high as 10-4
cm2V-1S-1. X-ray and UV analysis indicate that
PDI p-stacks
exist in these amorphous glassy solids and more importantly, both the
intra-stack separation and the extent of stacks can be systematically
altered. This is the first time the
degree of short-range order can be systematically controlled in a molecular
glass. The availability of this
adjustability may lead to many new technologically useful molecular glassy
materials.
4. Both graduate and undergraduate students were involved
in this project. One undergraduate
student carried out the related research in summer 2008 for three months. Five graduate students have been directly or
indirectly supported by this grant. This grant definitely impact them quite positively in terms of
career development.
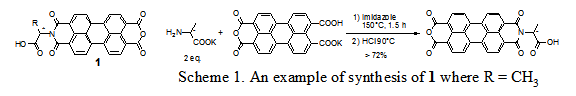
Copyright © American Chemical Society

