AmericanChemicalSociety.com
Reports: ND4 48781-ND4: Mechanism of Aldehyde Decarbonylase, a Novel Enzyme Involved in Hydrocarbon Biosynthesis
E. Neil G. Marsh, M.A., Sc.D., University of Michigan
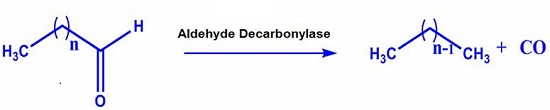
A promising route for the generation of new sustainable biofuels is to adapt biosynthetic pathways that directly produce fuel grade hydrocarbons. Although such pathways has been known for a long time, the enzymes that comprise them have not been studied to a large extent (1,2). The cer1 gene from A .thaliana has been proposed to encode an aldehyde decarbonylase protein that can convert long chain aldehydes to long chain alkanes (1). Very recently, another enzyme from cyanobacteria has been shown to possess aldehyde decarbonylase activity towards long chain aldehydes (3). This is a very unusual reaction, and a detailed mechanistic investigation of these enzymes may facilitate engineering of the enzymes to produce shorter chain, fuel grade hydrocarbons,
Studies
on Cer1 Protein Our studies have
been directed toward expression and mechanistic studies of these two
enzymes. Previous genetic studies
strongly implicated the cer1 gene
from A. thaliana as an aldehyde
decarbonylase (2). cer1 encodes a 67 kDa integral membrane protein that has an 8-His iron
binding motif common to membrane-bound diiron desaturases, which include fatty
acid desaturases and alkane hydroxylases (4). Attempts to overexpress the A. thaliana gene proved problematic, so
a synthetic gene was designed to include optimized codons for yeast and E. coli, affinity purification using a
His-tag and antibody recognition motifs. Since Cer1 is a eukaryotic membrane
protein, attempts for expression were first made to express it in yeast (S. Cerevisae). Standard yeast expression vectors did not yield any detectable
band in western blotting analysis, presumably due to very low expression
levels. Efforts are currently being made to increase the expression levels in
yeast.
Initial attempts
to express cer1 gene from pET28
vector in E. coli were not
successful. This might be due to the fact that E.Coli does not have the proper machinery to target Cer1 protein to
the membrane for proper folding, resulting in protein degradation inside the
cell. To overcome this problem, fusion protein partners were used. These fusion
partners include mystic protein and maltose binding protein, which were shown
to successfully target their N-terminal fusions to the membrane. Western blot
analysis showed, Cer1 protein can be over-expressed at high levels in fusion to
both mystic and maltose binding proteins, although presence of lower molecular
weight bands indicates proteolytic degradation.
Currently we are exploring assay conditions for Cer1. Since this enzyme is an 8-His motif
containing diiron enzyme, we will also examine the possibility that the enzyme
has a desaturase or hydroxylase activity.
Studies
on ADC Protein It was recently
shown that cyanobacteria possess an enzyme that converts long chain aldehydes
to alkanes and alkenes (3). The sequence similarity of this cyanobacterial
aldehyde decarbonylase protein (CAD) to ribonucleotide reductases suggested
that this protein is a member of the nonheme diiron superfamily. In fact, the
crystal structure of an ortholog of CAD from Prochlorococcus marinus has been solved as part of a structural
genomics effort without any assigned function. This is a soluble protein is
similar to the R2 domain of E. coli
ribonucleotide reductase, with a non-heme diiron center at the active site
ligated by histidine and carboxylate residues. A synthetic gene encoding this
protein was used to over-express the CAD in E.
coli and it has been purified in quite high yields (~150 mg/L). CAD as
purified has a broad UV absorbance at 350 nm, typical of non-heme proteins due
to a histidine-to-metal charge transfer band. Initial spectroscopic studies
showed that the protein is ~30% iron loaded as purified. Treatment of the
protein by high concentration of EDTA did not decrease the iron content,
suggesting that the iron is tightly bound. Expression in the presence of
ferrous ammonium sulfate increased the iron content of the protein by ~50 % as
purified. Current studies are focused on
the conditions need to activate the protein for activity. Future studies will focus on the metal
requirement, the possible involvement of molecular oxygen in the reaction and
the substrate specificity of the enzyme.
References 1. Aarts,
M. G., Keijzer, C. J., Stiekema, W. J., and Pereira, A. (1995) Molecular
characterization of the CER1 gene of arabidopsis involved in epicuticular wax
biosynthesis and pollen fertility, Plant
Cell 7, 2115-2127. 2. Cheesbrough,
T. M., and Kolattukudy, P. E. (1984) Alkane biosynthesis by decarbonylation of
aldehydes catalyzed by a particulate preparation from Pisum sativum, Proc Natl Acad Sci U S A 81, 6613-6617. 3. Schirmer,
A., Rude, M. A., Li, X., Popova, E., and del Cardayre, S. B. Microbial
biosynthesis of alkanes, Science 329, 559-562. 4. Shanklin,
J., and Cahoon, E. B. (1998) Desaturation and Related Modifications of Fatty
Acids1, Annu Rev Plant Physiol Plant Mol
Biol 49, 611-641
Copyright © American Chemical Society

