AmericanChemicalSociety.com
Reports: AC1 47942-AC1: Synthesis and Application of C2-Chiral Phosphinines to Asymmetric Catalysis
Charles M. Garner, Baylor University
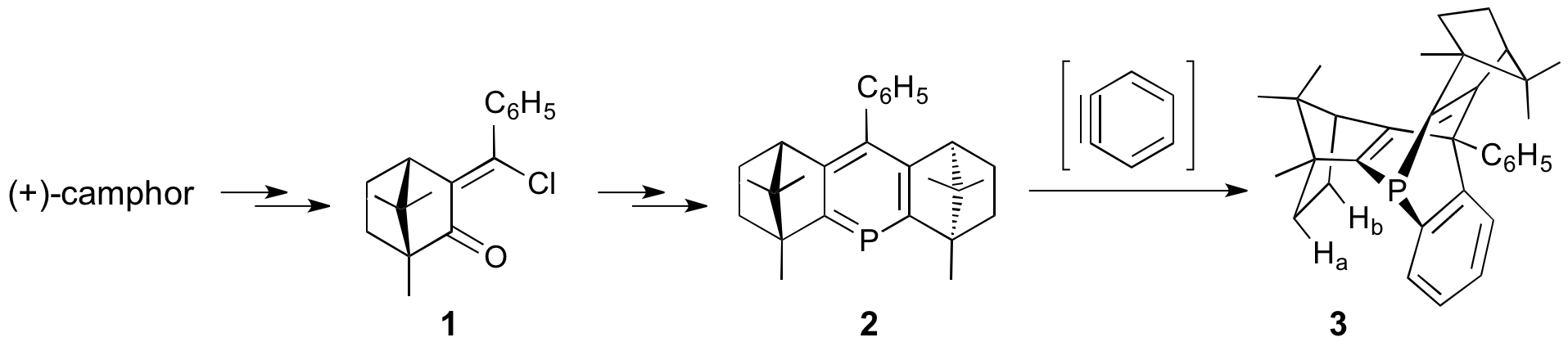
My laboratory has continued to study the preparation of unusual phosphorus ligands that are derived from chiral pyrylium salts. We have studied the conversion of chiral phosphinine 2 to the corresponding phosphabarrelene (3) via reaction with benzyne made by a few different methods. The phosphabarrelene, for which we have x-ray structural confirmation, exhibits remarkably upfield (+0.07 and -0.19 ppm) NMR signals for two of the protons (labeled a and b). Optimization of the yield is proceeding. Coordination of this to transition metals, and preparation of bis-phosphabarrelenes is in progress. Attempts to prepare chiral pyryliums (phosphinine precursors) from intermediate 1 have been thwarted by its low reactivity; under forcing conditions, any ketone enolates that can undergo aldol condensation do so in preference to the desired 1,4-addition. We have found that substituting a CF3 group for the C6H5 results in a more reactive intermediate, and we are pursuing new ligands on that basis.
Copyright © American Chemical Society