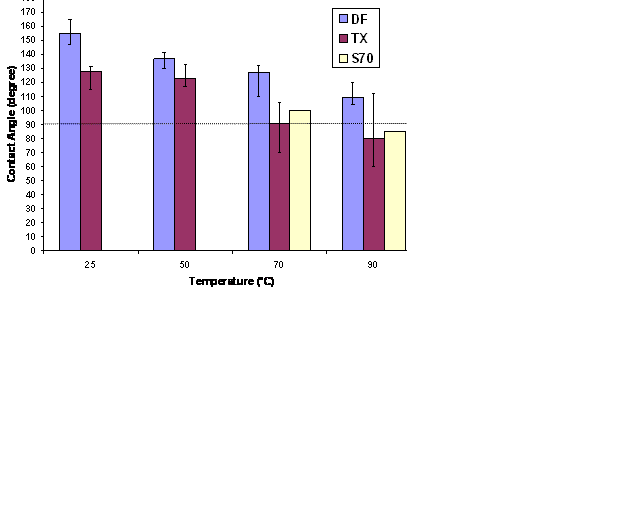AmericanChemicalSociety.com
Reports: AC9 46419-AC9: Mechanistic Understanding of Wettability Alteration in Fractured Carbonate Reservoirs
Kishore K. Mohanty, University of Texas (Austin)
The goal of this proposal is to develop a mechanistic understanding of the effect of potential determining ions, surfactants, oil composition, and temperature on the wettability of carbonate reservoir minerals. This knowledge can be used to alter wettability of initially oil-wet fractured carbonate reservoirs to recover oil from the matrix blocks. Wettability alteration at high temperature and high salinity is reported here. Modeling of the imbibition process would be studied in the next year.
Wettability alteration of a calcite plate can depend on parameters like brine salinity, oil composition, type of surfactant, concentration of surfactant, and temperature. Most of these experiments are done with a model oil (1.5 wt% cyclohexanepentanoic acid in n-decane). Adsorption and desorption of crude oil components (e.g., naphthenic and carboxylic acids) alter the wettability of mineral surfaces. Temperature can have an effect on both oil/water and oil/water/mineral interfaces. The adsorption of naphthenic acids and other carboxylic acids is believed to be a chemisorption type of reaction affected by temperature.
Figure 1: Effect of temperature on contact angle for anionic surfactants
The final contact angles for three surfactants (DF, TX, S70) at their optimal salinities for four temperatures (25 oC, 50 oC, 70 oC and 90 oC) are reported in Fig. 1. The wettability varies along the surface of the plate because of the non-uniformity in initial oil contact with the plate. This leads to a range of contact angles observed after surfactant treatment. The error bars in Fig. 1 indicate the variation of contact angle between different drops in the same experiment. At a very low surfactant concentration (0.1 wt%), intermediate to water-wettability can be obtained for many of the surfactants studied. The contact angle was found to decrease with increase in temperature for all the surfactants. For these surfactants the calcite plate remained oil-wet at 25oC and 50 oC, but altered to slightly water-wet at 70 oC and 90 0C. S70 was found to alter the wettability the most among all the surfactants at 90 0C; the final contact angle was 80o.
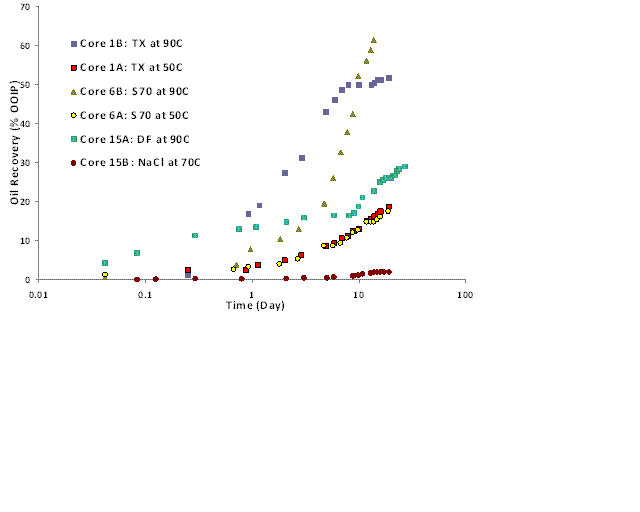
Figure 2: Oil recovery during imbibition with non-ionic surfactant solutions
Imbibition experiments were conducted by immersing initially oil-wet carbonate cores in these surfactant solutions. Fig. 2 shows the oil recovery in imbibition experiments for 10 md cores with these three anionic surfactants and a brine without any surfactant. The data plotted is an underestimate of the actual recovery because it does not account for the oil drops stuck to the core surface and the oil trapped in the macroemulsion (if any) below the separated oil layer. Brine without surfactant did not imbibe into the core; the oil recovery was less than 3% OOIP. TX and S70 recovered around 50% OOIP in about 10 days at 90 oC. Oil recovery for S70 exceeded 60% in about 30 days at 90oC. At 50oC, oil recovery is only 15% in 30 days, because the contact angles are high. Oil recovery for DF was about 30% in about 30 days at 90oC because the contact angle was not that low. Oil recovery generally increased as the temperature increased because the rock became more water-wet and oil viscosity decreased. The same trend was seen with nonionic surfactants.
Copyright © American Chemical Society