AmericanChemicalSociety.com
Reports: AC9 46928-AC9: Atomic Scale Simulation of Many Body Forces in Colloidal Nanoparticle Suspensions
Kristen A. Fichthorn, Pennsylvania State University
A quantitative understanding the forces between colloidal nanoparticles would benefit numerous applications in sensing, nanoelectronics, composite materials, nanofluids, optics, and catalysis that could exploit the unique properties associated with their small size. In applications involving crystallization, the aggregation of colloidal nanoparticles can be important and recent experiments have documented the phenomenon of oriented attachment, in which nanocrystals apparently approach one another and merge along specific crystallographic directions to form twinned or single-crystal structures [1-3]. The ability to direct crystallization through oriented attachment is an exciting prospect that could allow for the creation of new nanostructures with well-defined sizes and shapes. To realize the full potential of oriented attachment for achieving the controlled synthesis of a broad range of nanomaterials, insight into its origins, which are not currently clear in all cases, would be beneficial.
Progress in our group has been in two areas:
A. MD Simulation of the Aggregation of Titanium Dioxide Nanocrystals: Preferential Alignment[4]
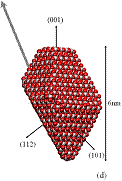
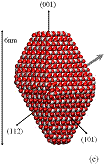
We used classical MD simulations to study the aggregation of various titanium dioxide (anatase) nanocrystals in vacuum. We consider charge-neutral nanocrystals with variations of the truncated tetragonal bipyramidal Wulff shape. Nanocrystalline anatase has been experimentally observed to possess approximate Wulff shapes [3]. The model nanocrystals are shown in Fig. 1, where we see that in addition to Wulff shapes, we consider asymmetric nanocrystals, which mimic possible off-Wulff shapes that could occur during crystal growth. The asymmetric nanoparticles possess permanent dipole moments, while the symmetric nanocrystals do not. Thus, we studied the effect of dipole-dipole interactions, which have been proposed to play a role in oriented attachment [1,2].
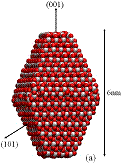
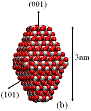
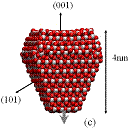
Figure 1: We studied (a)
large crystals with Wulff shapes; (b) small crystals with Wulff shapes; (c),
(d), (e) truncations of the Wulff shape, in which the relative magnitudes and
directions of the dipole moments are indicated by gray arrows. Oxygen atoms are red (dark) and titanium
atoms are white (light). All
the particles studied exhibit a strong tendency to aggregate with certain
preferred orientations in a ``hinge'' mechanism. Our simulations indicate that
electrostatic forces between under-coordinated atoms on the edges between
nanocrystal facets drive this phenomenon.
We find that aggregation rarely
occurs along the direction of the dipoles - even when the permanent dipole
moment is as large as 250 Debye.
Although the dipole-dipole interaction is the leading term in the
multipole expansion of the electrostatic potential for neutral particles,
higher order multipole moments (e.g.,
quadrupole, octupole, etc.) can also
contribute to the electrostatic potential.
Here, these high-order multipoles dominate the electrostatic potential
at short separations and they are created by regions of positive and negative
charge associated with under-coordinated surface atoms, as shown in Fig. 2. The observed mechanism for preferential
alignment may be a driving force for oriented attachment and the growth of
anisotropic structures during crystallization.
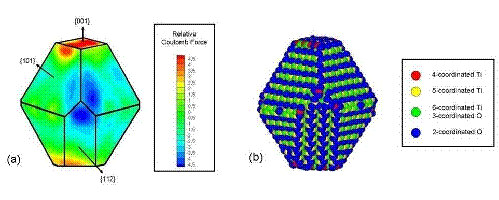
Figure 2: (a) Projected
map of the electrostatic potential in planes parallel to the nanocrystal facets
on the particle shown in Fig. 1(e). The
coordination numbers of the surface atoms are indicated in (b). Nanoparticle aggregation occurs between the red
(positively charged) and blue (negatively charged) regions shown in (a). B. Role of Solvent Ordering in Anisotropic Growth of Colloidal
Nanoparticles[5] Using molecular dynamics
simulations, we studied the role played by solvation interactions (interactions
arising from the ordering of solvent molecules around nanoparticles) in
influencing anisotropic growth of nanoparticles during crystallization. We
focus on the solvophilic system of Ag nanoparticles in an organic solvent. We
find that for the case of a mildly anisotropic nanoparticle, the ordering of
solvent molecules around it makes it more favorable for incoming nanoparticles
to aggregate on its smaller surfaces, further increasing the anisotropy of the
original nanoparticle. Our results show that solvation forces could play an
important role in the anisotropic growth of colloidal nanoparticles. References 1. Niederberger,
M.; Colfen, H. Phys. Chem. Chem. Phys.
2006, 8, 3271.
2. Zhang,
Q.; Liu, S.-J.; Yu, S.-H. J. Mater. Chem.
2009, 19, 191.
3. Penn,
R. L.; Banfield, J. F. Geochim.
Cosmochim. Acta 1999, 63, 1549.
4.
Alimohammadi,
M.; Fichthorn, K. A. Nano Letters 2009, 9, 4198. 5. Sathiyanarayanan, R.; Gergidis, L.; Fichthorn, K. A.
(In preparation).
Copyright © American Chemical Society