AmericanChemicalSociety.com
Reports: AC1 46200-AC1: Catalyst Design and Development: Exploration of a New Class of Rhodium (I) Catalysts
William C. Trenkle, Brown University
Catalyst design and development: Exploration of a new class of Rhodium(I) catalysts
The formation of carbon-carbon bonds is a critical operation in the assembly of molecules. Numerous groups are engaged in the development of new, or improved, procedures for this purpose. Organometallic catalysis allows for the discovery and elucidation of new reaction pathways to accomplish the overarching goal of bond formation. Our group is motivated by the thesis that these limits can be overcome with a new class of multifunctional rhodium(I) and iridium(I) quinonoid catalysts. Remarkable features of the rhodium catalyst system includes tolerance of extremely electron deficient aryl boronates (including the first example of trihalogenated substrates) as well as ability to catalyze the formation of quaternary carbon centers through addition to beta,beta-disubstituted conjugate acceptors.
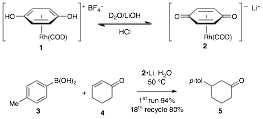
Previously, we have published that highly water soluble rhodium(I) quinonoid catalysts (such as 2¥Li) can be prepared through simple combination of the precatalyst with aqueous solutions of metal hydroxides (Li, K, Na) to form the a solution of the active catalyst. The organometallic species has been characterized spectroscopically and is remarkably stable in aqueous solution. This solution successfully catalyzed conjugate addition of aryl boronic acid 3 to enone 4 and was recycled 18 times with high efficiency (~95% yield first cycle and 85-80% for runs 10-18).
Additionally, we disclosed that immobilization of
quinonoid Rh catalysts on silica-gel
was accomplished by a Surface Sol-Gel process. This solid supported catalyst
system gives ca. 2 times higher yield than free quinonoid Rh
catalysts (1 and 2K+) in polymerization of phenylacetylene (6)
to polyphenylacetylene (7). Due to the surface structure of monolayered
silica gel Rh catalysts, shorter length polyphenylacetylenes were produced in comparison to those
obtained with the insoluble 2 and 3K+. The silica-gel supported catalysts
produced polymers having comparatively constant polydispersity
regardless of the amount of catalyst or the solvent utilized during catalysis.
Present work in our group continues to be focused on the formation of hetero
bi-layer catalysts on a silica-gel surface with arene
metal complexes having di-functional groups. We have also revealed the preparation,
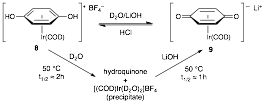
characterization and evaluation of an iridium(I)
quinonoid catalyst (9). This catalyst is similarly water-stable
and soluble. It also catalyzes the
one of the first reports of Iridium catalyzed conjugate addition of aryl
boronates to a conjugate acceptor.
Studies continue to fully evaluate the activity and reactivity of this
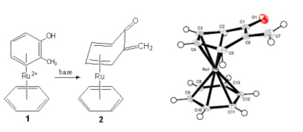
class of catalysts. Most recently, we have found that ortho-hydroxy-toluene
can undergo a similar coordinative deprotonation in the presence of ruthenium(II) to produce the corresponding ortho-quinone methide (QM). The QM structure is known as an
independent molecule but, in contrast to quinones,
these are extremely unstable, especially when unsubstituted. QMs have been
previously stabilized by attachement of Rh, Ir, and Pd transition metal
fragments, but this is the first ruthenium-stabilized QM and the first unsubstituted QM complex to be characterized crystallographically.
We are currently exploring the reactivity and stability of this new
class of complexes. In summary, during the exploration of this new class
of catalysts, we have recently discovered that rhodium(I)
quinonoids are recyclable catalyst systems that catalyze the selective addition
of aryl and vinyl boronic acids to conjugate acceptors, elucidated the
structure and preliminary activity of a new class of iridium(I) quinonoids and
ruthenium quinone methides,
as well as a method for the highly stereoselective
polymerization of phenylacetylene with solid
supported rhodium(I) quinonoids.
The funding from the PRF has supported this work and allowed it to be
published.
Copyright © American Chemical Society