AmericanChemicalSociety.com
Reports: GB6 48651-GB6: Ab Initio Studies on the Catalytic Roles of Platinum-Doped Carbon Nanotubes in Fuel Cell Electrodes
Hee-Seung Lee, University of North Carolina (Wilmington)
In the field of energy
technology, proton exchange membrane fuel cell has been the front runner for the
environmentally friendly power source. However, the widespread use of fuel
cells has been hampered by the high cost of metal catalysis, such as platinum
(Pt). To develop cost-effective fuel cell systems, it is highly desirable to
maximize the efficiency of Pt catalyst or replace it with cheaper solutions. Given
recent suggestions from numerous experiments studies that Pt-doped carbon
nanotubes (Pt-CNT) have better performance over the conventional carbon black
based systems, we carried out electronic structure calculations to understand
the electronic properties and catalytic effects of Pt-CNT. During the past grant year, we have completed the electronic
structure calculations of some representative Pt clusters adsorbed on (5,5)
single-walled nanotube (SWNT), up to four platinum atoms, and started
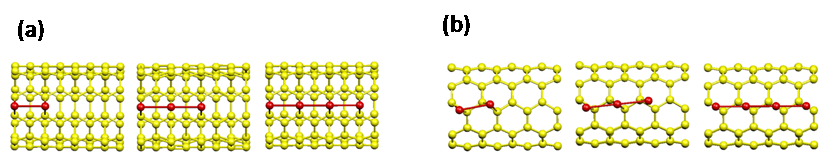
investigating oxygen dissociation reactions on the Pt-CNT system. Pt-monomer/CNT: There are three distinct adsorption sites for Pt
on CNT: on-top site (above carbon atom), hole site (above C6
honeycomb), and bridge site (above C-C bond). Calculations showed that the
bridge sites have much higher binding energies than others. Bridge sites can be further classified into
"orthogonal" (B1, Fig. 1(left)) and "skewed" (B2, Fig.1(right))
sites. The Pt-C bond lengths are 2.06Å for B1 site and 2.08Å for B2 site. These
bond lengths are more compatible with van der Waals interaction than covalent
interaction.
However the binding energies of Pt 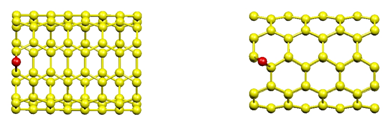
Fig.1 Single platinum
adsorbed on "orthogonal" site (B1, left) and "skewed" site (B2, right) of (5,5)-SWNT
Linear Pt Chain/CNT : We investigated the geometries and electronic
structures of a wide variety of Pt-clusters absorbed on (5,5)-SWNT to
understand the nature of Pt-C interaction as well as the role of Pt-Pt
interaction in the formation of Pt-CNT. For example, we studied the linear chains of Pt clusters adsorbed on CNT,
starting from Pt dimer to Pt tetramer. Due to the size of our system (four CNT
unit cell), a linear Pt tetramer on (5,5)-SWNT forms a infinite Pt chain. We
included both linear Pt-chains adsorbed on the B1 and B2 sites in our
calculations (see Fig. 2). Fig. 2 Linear platinum chains
on (5,5)-SWNT : (a) B1 site, and (b) B2 site.
For the Pt dimer and trimer on B2 site, it was found
that Pt chains are not parallel to the CNT axis, but slightly rotated. This
increases the Pt-Pt bond length of dimer on B2 site to 2.63Å. As the number of
Pt increases, Pt-Pt distance decreases and reaches 2.5 Å when the chain becomes infinitely long
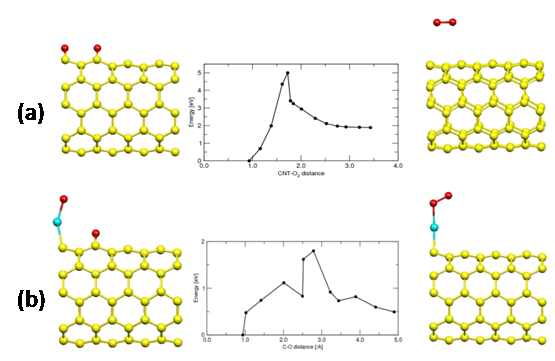
Oxygen dissociation on Pt-CNT : To understand the catalytic role of platinum on the surface of nanotube, we began investigating the oxygen dissociation reaction on the surface of (5,5)-SWNT with and without platinum clusters. Since the catalytic activity of CNT doped with Pt cluster is expected to change drastically depending on its geometry and electronic properties, a variety of Pt clusters adsorbed on (5,5)-SWNT are currently under investigation. As an example, we are reporting oxygen dissociation on the B1 site of (5,5)-CNT doped with a single Pt atom. The adsorption of O2 on a pristine SWNT is quite weak and the oxygen has to change its electronic structure from triplet to singlet state upon dissociation. The activation barrier of this reaction on the B1 site of pristine (5,5)-SWNT (Fig. 3a) was found to be 3.0 eV. The triplet-singlet transition occurs right before the bond dissociation. On the other hand, the barrier height for the same reaction on the B1 site of Pt-doped (5,5)-SWNT is only 1.3 eV, which is still high, but much lower than on the pristine (5,5)-CNT. The dissociation actually occurs on the single Pt atom and the oxygen atom migrates to a nearby B1 site (Fig. 3b). The activation barrier is expected to decrease as the size of Pt cluster increases.
Fig. 3 Reactant (right) and product structure (left) for the oxygen dissociation on a (a) pristin (5,5)-SWNT and (b) Pt-doped (5,5)-SWNT. The energy level diagram is include in the middle. Oxygen atoms are in red and platinum atoms are in cyan color.
Copyright © American Chemical Society