AmericanChemicalSociety.com
Reports: UNI6 49527-UNI6: Fundamental Studies of Atmospheric Pressure Microhollow Cathode Discharge Plasma Jet Interaction with Liquid Media
WeiDong Zhu, Saint Peter's College
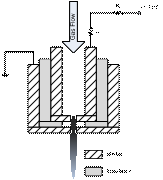
The MHCD based PMJ device
comprises two metal tubes separated from each other by a third insulating tube.
The key dimensions are the inter-electrode distance and the diameter of the
exit nozzle, which are 0.5 mm and 0.4 mm (or 0.8 mm), respectively. The
electrode embedded in the device is connected to a DC high voltage power supply
while the outer electrode is grounded for safety considerations. A ballast
resistor, followed by a current monitoring resistor is inserted between the
powered electrode and the DC power supply.
The inner tube also serves as the channel for the gas flow to the
discharge area. Gas flow rate
usually ranges from 0.3 to 5 standard liters per minute. The discharge
sustaining voltage varies in the range of 230 ¨C 600 V (depending on the working
gas used) with an operating current in the 3 ¨C 40 mA range. A schematic diagram of the essential part
of device is shown in figure 1 (a).
(a)
(b)
Figure 1. (a) A
schematic diagram of MHCD plasma microjet device; and (b) air PMJ working in
water
There are two ways the PMJ
can interact with liquid media: (1) PMJ sustained in air, in close contact with
liquid; and (2) PMJ sustained in quasi-steady gas cavity in water (A picture of
this situation is shown in figure 1 (b). Nevertheless, either way, a stable PMJ
has to be generated in air before the interaction with liquid happens. Initially,
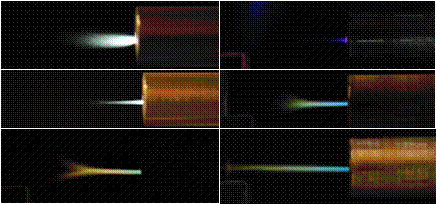
room air was compressed, dried and used as working gas. Figure 2 shows two
examples of the PMJ working in air. The PMJ produced varies in length and
diameter, depending on the diameter of the exit nozzle and the gas flow rate.
Both laminar (figure 2, left top) and turbulent (figure 2, left middle) mode of
the jet were observed. The length of the jet is ranging from 0.5 ¨C 1 cm. Not to
my surprise, a considerable amount of ozone (tens to a few hundred ppm,
measured by an ozone analyzer) was produced since all the conditions are right
for the production of ozone with air present. Besides, the PMJ can get quite
hot. The surface of the outer electrode reaches about 70 oC,
although at 1 cm away from the exit nozzle, the temperature is closer to room
temperature due to the forced flow cools the system down. Helium and
helium/oxygen are used in some cases to replace air to generate
different type of reactive species as well as to reduce downstream temperature.
Figure 2 also shows three pictures of the helium or helium/oxygen PMJ. When running with pure helium, the
visible part of the PMJ is very faint, a short bluish jet was observed (figure
2, right top). Upon mixing oxygen in the gas, the PMJ becomes much better
defined. The visible jet length increases tremendously when oxygen is added
into the helium stream, reaching 30 mm in the laminar mode (figure 2, right
bottom). At higher flow rate, turbulent mode (figure 2, right middle) is
observed. At higher operating currents (I > 25 mA), the temperature of the
jet at 1 cm away from the exit nozzle as well as the temperature of the outer
metal tube remained approximately the same as the air as working gas situation,
but can be decreased to a lower value by lowering the discharge current. At a
current of 5 mA (average power consumption around 2-3 W), with an oxygen
concentration above 0.1%, a visible jet can still be achieved, rather with a
shorter jet length (around 1 cm). In
a particular condition, bifurcation of the jet occurred (figure 2, left bottom)
Figure 2.
Pictures of PMJ working in air and in helium or helium/oxygen mixture
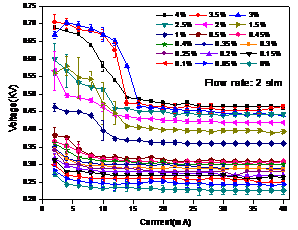
Current-voltage
curve of the PMJ exhibits a self-pulsing mode (characterized by a negative
differential resistance, NDR) followed by a normal glow mode. Figure 3 shows an
example of the I-V characteristics when helium/oxygen (0-4%).
Figure 3. I-V characteristics of PMJ
with helium/oxygen (0-4%) as working gas
When the PMJ was injected into water, a set of fixed
parameters were chosen to maintain a stable operation (Current: 30 mA, flow
rate: 2 slm, water volume: 20 ml).
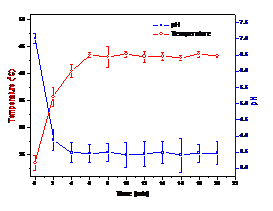
Basic water properties such as pH and overall temperature were
monitored. When air is used as
working gas, pH of water (distilled) drops to and stabilizes at around 3.5 in 2
minutes. Overall water temperature increases from room temperature to
approximately 42 oC in 6 minutes. Figure 4 shows the time evolution
of the pH and water temperature. When helium or helium/oxygen (2%) is used as
working gas, the pH barely changes to about 6, probably due to the mixing-in of
air above the water. Water temperature is slightly lower than in air case,
reaching around 37 oC.
Figure 4. PH and overall temperature change of 20 ml distilled
water with air PMJ treatment
(a) (b)
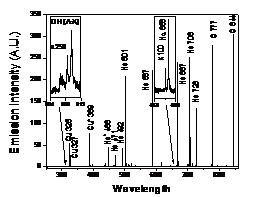
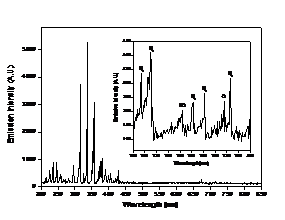
Figure 5. Optical emission spectra of PMJ with (a) air and (b)
helium/oxygen (2%) as working gas
Future work includes detailed study of the PMJ interaction
with contaminated water, analysis of radicals and ions generated in the water.
 Optical
emission spectra of the air and helium/oxygen were taken with an intensified
CCD through a monochromator. In air case, majority of peaks are from nitrogen
emissions with weak oxygen emission at 777 nm. However, when helium/oxygen is
used as working gas, helium emissions dominate the visible spectrum. However,
strong oxygen emissions at 777 nm and 844 nm are observed. When varying the
oxygen concentration in the working gas, it is found that the oxygen emissions
peak at an oxygen concentration of 0.1-0.2%. This is attributed the penning
ionization of oxygen by helium metastables followed by an electron impact
dissociation. Figure 5 shows the emission spectra of PMJ with (a) air and (b)
helium/oxygen (2%) as working gas.
Optical
emission spectra of the air and helium/oxygen were taken with an intensified
CCD through a monochromator. In air case, majority of peaks are from nitrogen
emissions with weak oxygen emission at 777 nm. However, when helium/oxygen is
used as working gas, helium emissions dominate the visible spectrum. However,
strong oxygen emissions at 777 nm and 844 nm are observed. When varying the
oxygen concentration in the working gas, it is found that the oxygen emissions
peak at an oxygen concentration of 0.1-0.2%. This is attributed the penning
ionization of oxygen by helium metastables followed by an electron impact
dissociation. Figure 5 shows the emission spectra of PMJ with (a) air and (b)
helium/oxygen (2%) as working gas.
Copyright © American Chemical Society