AmericanChemicalSociety.com
Reports: ND5 49114-ND5: Direct Measurement of the Force between Neutral Dipolar Materials Induced by External Electrostatic Fields
William A. Ducker, Virginia Polytechnic Institute and State University
The forces acting between small particles (colloids) control the properties of many materials, such as minerals, paint, soils, ceramics during processing, and dairy products. Forces such as the van der Waals force, double-layer force, hydration, depletion, and polymer steric forces have been widely studied using the Surface Forces Apparatus, the Atomic Force Microscope (AFM), and other techniques.
In this project, we have investigated the dipolar force that acts between two particles in the presence of an electric field. In air or a low-dielectric material, this dipolar force is very large in magnitude compared to all forces other than gravitational forces. It is therefore very important in the processing of dry minerals, printing, electrorheological fluids, and the processing of oil. The objective is to make accurate measurements of the force as a function of separation between dielectric solid colloidal particles in air and oils, and to compare these measurements to theoretical predictions.
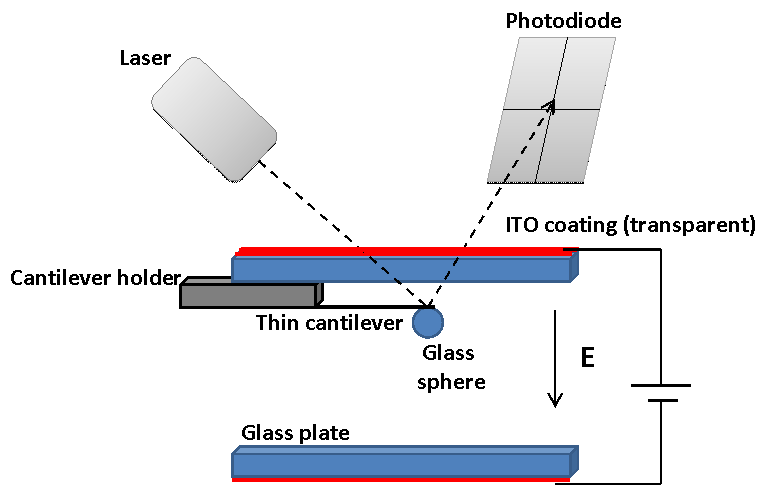
To measure the force as a function as a function of separation for a particle and a plate, we have introduced electrodes into an AFM (shown schematically in Figure 1) and attached a particle to the force-measuring cantilever. To measure the force between two particles, we simply attach a particle to the bottom plate.
Figure 1: Schematic of experiment to measure the force
between particles in an electric field.
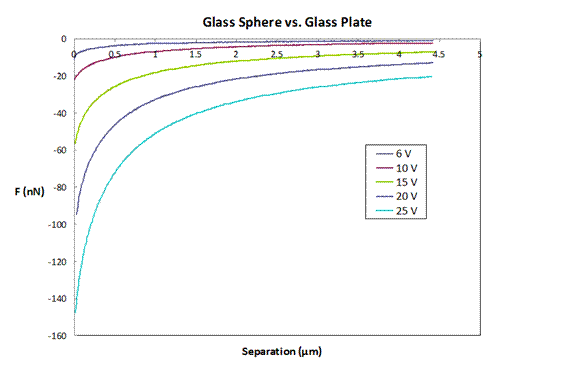
Figure 2: Force as a function of applied voltage.
Results of force measurements as a function of applied
voltage between the plates are shown in Figure 2. The measured forces are much larger than the
van der Waals force, and increase with the applied field, as expected. The applied force should scale with the
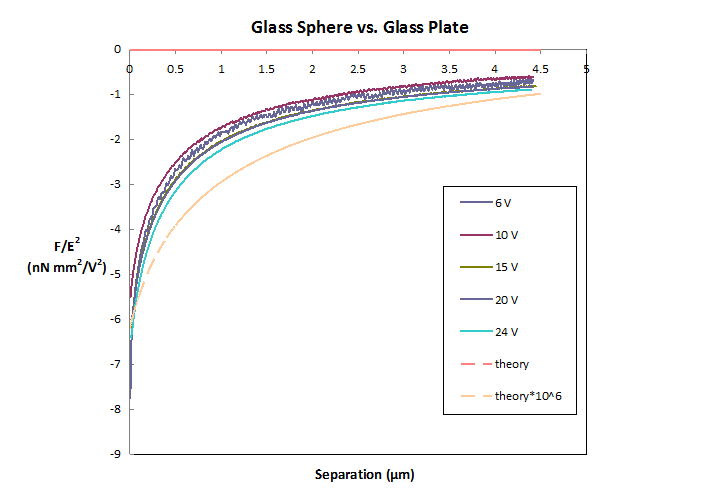
square of the electric field, and the scaled data is shown in Figure 3.
Although there is some variation, the measured force is seen to scale
approximately with the applied field.
However, there is one very surprising effect: the measured force is much
larger in magnitude than expected. Figure 3 also shows the theoretical force,
calculated by Cox, and that force multiplied by about 106. Clearly the measured force is about 106
times larger than the measured force.
Figure 3: Measured force scaled by the square of the applied
electric field.
Much of our effort has been devoted to understanding why the
measured force is so large. To date, we
have determined two significant clues. First,
in the absence of any field, the force shows evidence of the formation of a
capillary bridge, which is probably due to condensed water. We know that that forces in the presence of
the field are much larger than, and the incorrect functional form for a
capillary force. But the presence of a
water film could cause a significant change in the polarization force because
the water probably contains conducting ions. Second, the force depends on the
time during which the field is applied.
This may also be due to conduction of charge from the measurement
cantilever.
Thus our ongoing effort focuses on the alteration of our
experiment to exclude a thin water film from around the particles and
plates. We will then measure the force
as a function of water film thickness. When the film has zero thickness, we
should be able to measure the “pure” dipolar force, and at high humidity, the
conduction plus dipolar force.
Copyright © American Chemical Society