AmericanChemicalSociety.com
Reports: ND3 48822-ND3: pi-Bonded Cationic Ligands as Catalysts and Precursors
Richard Kemp, PhD, University of New Mexico
Our recent ACS-PRF Type ND grant is geared towards the synthesis and characterization of a new class of ligand to be used in the preparation of organometallic complexes. Decades of research in inorganic chemistry have shown that there exists no more critical interaction when preparing a metal-based complex or catalyst than the metal’s σ or π bonding interactions with its framework ligands. Certain ligands are effective in particular applications while others are not. However, the ligand toolbox that has been used to date to prepare new compounds is composed essentially entirely of either neutral or negatively-charged ligands. While there exist σ-binding cationic ligands, surprisingly, positively-charged ligands that can π bond to metals, as do many commonly-used anionic ligands, are to our knowledge completely unknown. Our research efforts are directed towards the preparation of novel cationic ligands and to examine their reactions towards metals. The ligands that we have targeted are based on [(R2P)3C]+ or [(R2N)3C]+ tripodal frameworks. Previous work has shown that the anionic versions of these ligands can exist and ligate to metals. We postulate that these carbocation ligands will be stabilized by pπ-pπ bonding from the adjacent N or P heteroatoms. Hence, in properly-designed systems, we anticipate that these cationic ligands will bind to metal precursors to form new, charged complexes. We have a unique opportunity to invent a new family of ligands and to study them theoretically and experimentally. Most importantly, we have a possible opportunity to fundamentally alter the manner in which synthetic chemists think about ligand-metal interactions.
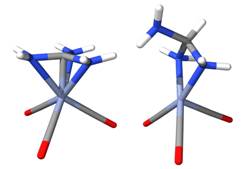
Prior to beginning experimental work we needed to establish whether this overall concept was feasible computationally. Our preliminary density functional theory (DFT) calculations have indicated that the central C atom in each of these free ligands is planar, with shortened C-P or C-N bonds in the cation indicating an increased level of pπ-pπ bonding in the cation. Most importantly, computational results have indicated that there exists a net positive bonding between these cations and various organometallic fragments. The net positive bonding case is shown in Fig. 1 for the model system {[(H2N)3C]Cr(CO)3}+ (A) and its uncharged parent complex [(H2N)3CH]Cr(CO)3 (B). Note that the uncharged parent complex B is calculated to be a di-hapto σ-bonded complex while the cation A is calculated to be a three-fold symmetric π-bonded complex. The N-C-N bond angles in the complex are approximately 120°, showing a significant flattening from the precursor. As well, the three C-N bonds in the cationic complex are noticeably shortened relative to the uncharged parent complex, again indicating a net attraction for a π-bonded complex to a metal-containing fragment.
Fig.1 Comparison of the Structures of A and B via DFT.
Most of our
synthetic efforts during the initial period of funding has focused on the
attempted preparation of these free [(R2N)3C]+
and [(R2P)3C]+
ligands. In order to accomplish
this one must formally remove a hydride from the (R2N)3CH or (R2P)3CH starting
materials. While this is easy
conceptually to do on paper, we are finding this task to be a very challenging
step in reality. Our initial attempts
have involved the use of strong Lewis acid reagents to remove the hydride. Use of acidic reagents such as HBF4, [Ph3C][BArf]
(BArf = perfluorinated tetraphenyl borates), BPh3, AlMe3,
B(C6F5)3, SbF5, TaF5,
and the like have not as yet shown the ability to remove hydride from the
starting (R2N)3CH or (R2P)3CH
species. The lack of hydride reactivity
has been surprising to us and is quite problematic. In many of the reactions of
(R2P)3CH with highly-electron
deficient acids such as BF3, B(C6F5)3,
or SbF5, we have seen degradation of (R2P)3CH
into the bis-species, (R2P)2CH2.
Other P-containing materials are as-yet unidentified. However, we are in the
process of converting (R2P)3CH
into (R2P)3C-X species to provide a more effective halide
leaving group. Concurrent with the
preparations of free cationic ligands, we are also synthesizing metal complexes
of the parent (R2P)3CH and (R2N)3CH
ligands. We have attached these neutral
species to Group 6 metal carbonyl fragments to provide precursor
complexes. While this is not the
intended mode of complexation of the ligand, we will likely be able to use
these complexes prepared in a “roundabout fashion” to examine the stability of
the cationic metal complexes, as removal of the hydride from a metal complex is
expected to be easier than removal from a free ligand. Fig. 2
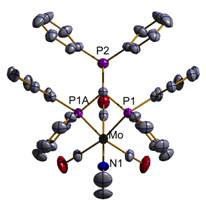
shows a crystal structure of an example of complexed di-hapto (Ph2P)3CH to a
M(CO)3(EtCN) fragment (M = Mo, W).
Addition of B(C6F5)3
leads to removal of EtCN and generation of the symmetrical, tri-hapto [(Ph2P)3CH]M(CO)3
complexes. Reactions to remove the
hydride from the complex are underway.
Fig. 2 X-ray
Structure of [(Ph2P)3CH]Mo(CO)3(EtCN). The granting of
this award from ACS-Petroleum Research Fund has been pivotal in allowing our
research group to expand into an entirely new direction. While the other research projects currently
ongoing in the group are directed towards specific targets, the long-term value
in this award is that it is directed towards exploring and developing a new
chemical concept that we believe will be useful in synthetic chemistry and
catalysis. As well, by funding his research this grant has allowed one minority
student, Mr. Wilson William, to complete his experimental laboratory work
towards his Ph.D. degree. The
opportunity to work on this grant for an initial summer was instrumental in
allowing another new graduate student, Mr. Christopher Larsen, to choose our
group for his graduate studies. During
this time, Mr. Larsen learned the synthetic techniques and skills that will be
required as a graduate student in synthetic organometallic chemistry. Lastly, a
postdoctoral student, Dr. Diane Dickie, has also been partially funded by this
ACS-PRF project, thus allowing her to develop her mentoring skills needed for a
possible academic career.
Copyright © American Chemical Society