AmericanChemicalSociety.com
Reports: DNI5 49041-DNI5: Molecular Modeling of Ionic Liquids Confined in Nanoporous Carbons
Francisco R. Hung, PhD, Louisiana State University
Abbreviations: dye-sensitized solar cell (DSSC), electrochemical double-layer capacitor (EDLC), ionic liquid (IL), molecular dynamics (MD), mean squared displacement (MSD), multi-walled carbon nanotube (MWCNT), nanoparticles (NPs), organic light-emitting diode (OLED)
During the past 17 months, my group has been focused on studying the structural and dynamical properties of several (ILs) confined inside nanoporous carbons using MD simulations. A fundamental understanding of the properties of ILs inside nanopores is relevant for applications of ILs as alternative electrolytes for energy storage in EDLCs. These devices have a structure similar to a battery, consisting of two carbon-based porous electrodes, an electrolyte and an ion-permeable separator. EDLCs cannot store as much energy as fuel cells and batteries, but exhibit fast charge/discharge times and can provide bursts of energy very quickly. As a result, EDLCs can complement the capabilities of internal combustion engines, fuel cells and batteries in hybrid vehicles, industrial equipment and electronic devices. Using ILs as alternative electrolytes allow EDLCs to operate at higher voltages, which results in improved energy storage and better charge/discharge times. ILs in contact with nanopores are also present in DSSCs. Therefore, a fundamental understanding of the behavior of ILs inside nm-sized pores is crucial for the rational design of EDLCs. In particular, the structural properties of the confined ILs affect the capacitance in EDLCs, and the dynamical properties of ILs inside nanopores is one of the main factors determining the electrical resistance in an EDLC.
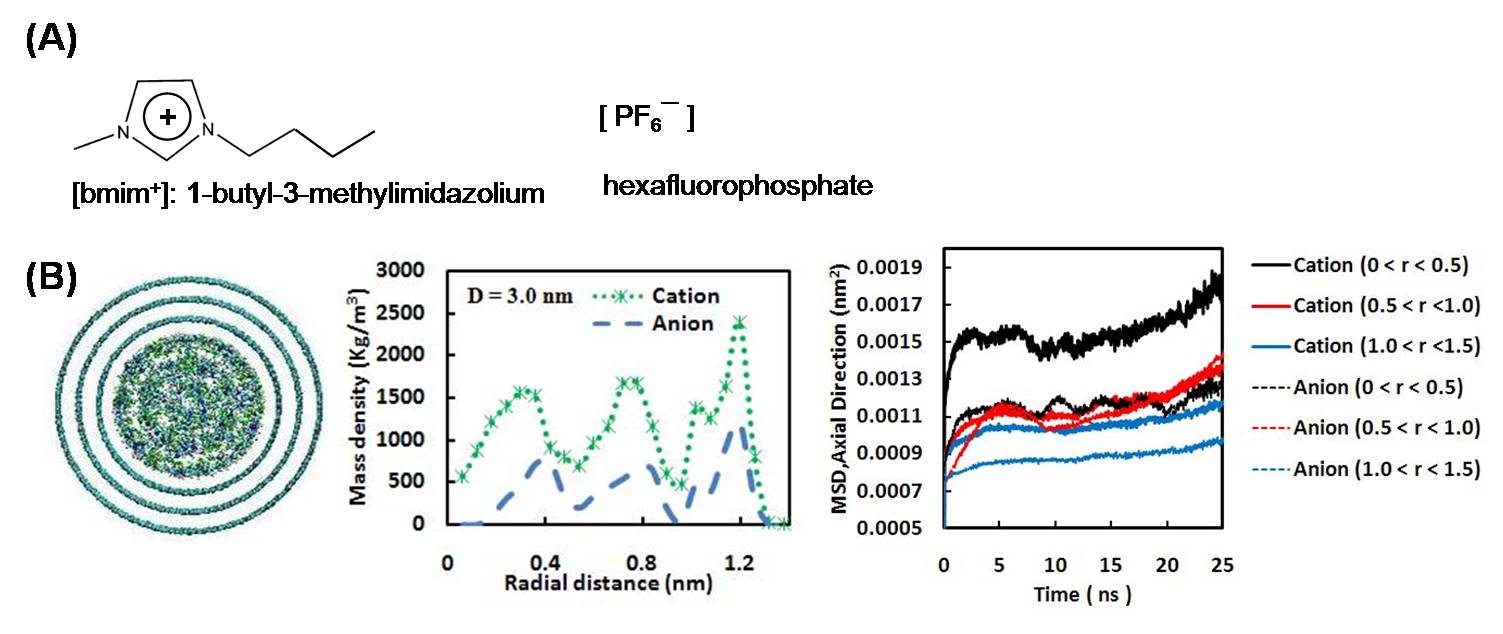
We have studied the dynamical properties of the IL [bmim+][PF6-] (Fig. 1A) confined inside MWCNTs with several pore sizes at 300 K via MD simulations. Variables such as pore size and pore loading (i.e., the amount of IL inside the MWCNT as compared to the bulk density of the IL) have a profound effect on the structure and dynamics of the confined IL. The ions tend to form concentric layers in the radial direction (Fig. 1B). The layering behavior depends directly on the pore size and the pore loading. At a pore loading ρ = ρbulk, the confined ions were found to have different mobilities according to their radial position (Fig. 1B). The dynamics are
Figure 1. (A) Scheme of the IL [bmim+][PF6-]. (B) MD simulations of [bmim+][PF6-] in a MWCNT, D = 3.0 nm, ρ ~ ρbulk. From left to right: snapshot, radial density profiles of the ions, and axial MSD for ions in different layers.
measured by the ions' MSDs in the axial direction as a function of time. Our results indicate that the ions in the center of the pore move faster than those in the middle layer, which in turn have faster dynamics than those ions near the walls. However, the dynamics are not completely regular. For example, the axial MSD of the anions in the inner and middle layers are similar in magnitude; in contrast, the cations in the middle layer move faster than those in the inner layer. Furthermore, the cations in the inner and outer layers have larger MSDs than the anions in those layers, but the MSDs of the cations and anions in the middle layers are comparable in magnitude. These results suggest that the local dynamics of ILs confined inside MWCNTs are very complex and heterogeneous.
In another series of studies, we have teamed with the group of Prof. Isiah Warner (Chemistry, LSU) to study the optical properties of IL-based NPs. These optical properties depend on the orientation of individual chromophores within the NP. Consequently, understanding the factors governing the assembly of these individual chromophores within a NP is crucial for the rational design of NPs with desired optical properties for applications ranging from OLED to photovoltaics to biomedical imaging. In our combined experimental-simulation study, we explored a new method to tune the spectral properties of fluorescent IL-based NPs through control of the aggregation behavior of cationic dyes. In these studies, a water soluble dye, 1,1',3,3,3',3'-hexamethylindotricarbocyanine iodide, [HMT+][I-], was subjected to ion exchange
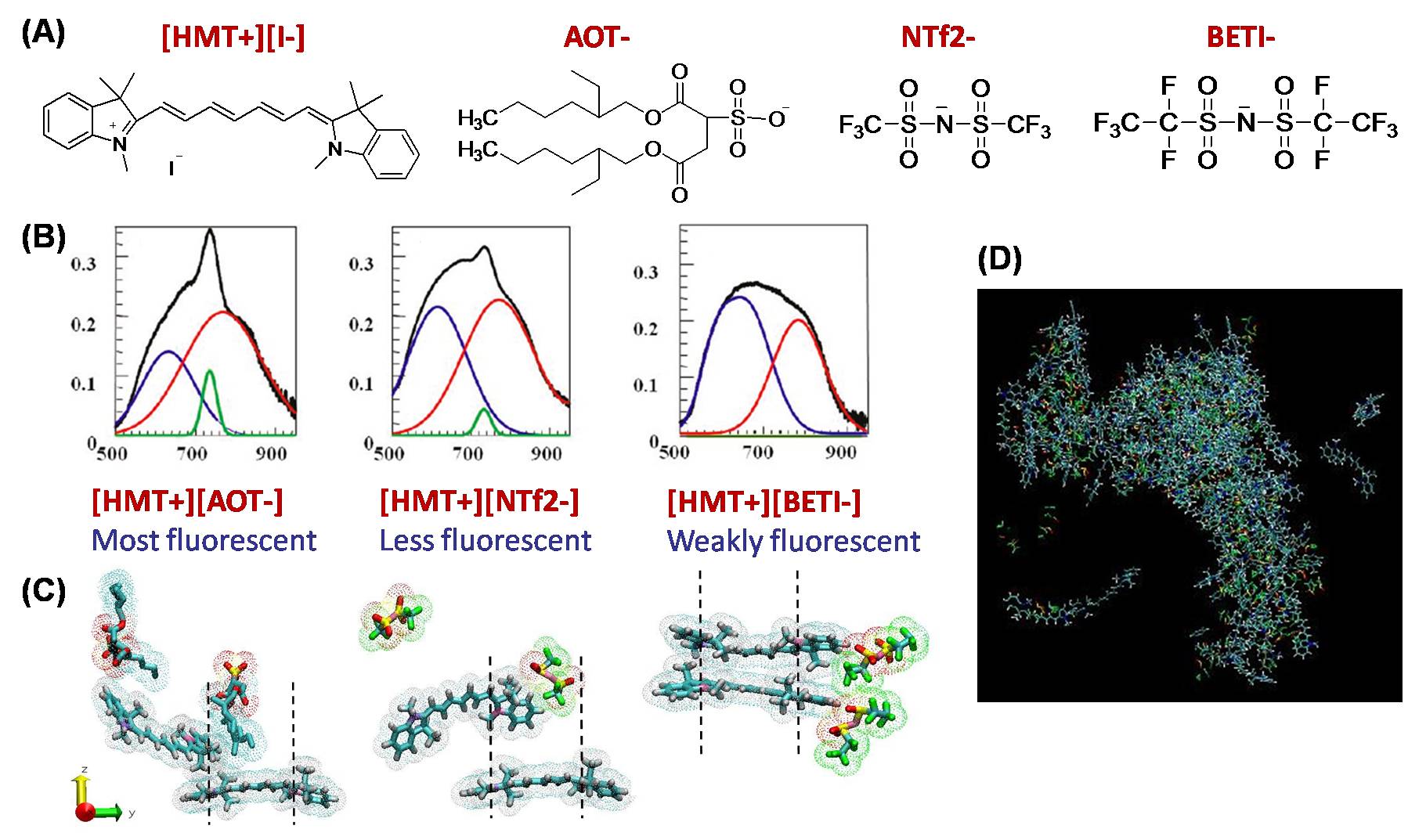
Figure 2. (A) Scheme of the cations and anions studied. (B) Total (black) and deconvoluted absorption spectra of dye NPs, showing contribution of H- (blue), random (green), and J- (red) aggregates. (C) Simulation snapshots showing J-aggregates for [HMT+][AOT-] and [HMT+] [NTf2+], and H-aggregates for [HMT+][BETI-] (D) Simulation snapshot of the earlier stages of the formation of a [HMT+][NTf2+] NP in water (box length ~ 20 nm)
with several anions, including bis(2-ethyl-1-hexyl) sulfosuccinate [AOT-], bis(trifluoromethane- sulfonyl) imide [NTf2-] and bis(pentafluoroethanesulfonimide) anion [BETI-] (Fig. 2A). All prepared HMT dyes were equally fluorescent in ethanol; however, when IL-based NPs were prepared in aqueous dispersion, different degrees of fluorescence are observed (Fig. 2B). Assisted by MD simulations (Fig. 2C), these observations were explained in terms of the aggregation behavior of the cations within the NPs. [AOT-] and [NTf2-] induced predominant J-aggregation of [HMT+] within the NPs (i.e., the cations form a brickwork or head-to-tail assembly); in contrast, [BETI-] induced a predominant H-aggregation of [HMT+] (i.e., card-pack assembly). MD simulations provided a molecular-level confirmation of these experimental observations. In Fig. 2B we present representative snapshots from MD simulations, showing the preferred stacking arrangements of two [HMT+] cations in the presence of different anions in aqueous dispersion (water molecules were not depicted for clarity). The trends observed in our simulations are in excellent agreement with the experimental observations. MD simulations of the early stages of the formation of the IL-based NPs (Fig. 2D) suggest that different types of aggregates (H-, J- and random) are observed within the NP, also in good agreement with experiments.
Monies from this grant have been used to partially support a postdoctoral researcher and a PhD student in my group. This student recently received a M.S. degree in Chemical Engineering while working towards obtaining his PhD degree. Support from this ACS-PRF grant has so far resulted in two publications and one additional manuscript recently submitted for publication. Our work has been also featured in seven presentations at scientific conferences (e.g., AIChE 2009 Annual Meeting, ACS Fall 2010 National Meeting).
Copyright © American Chemical Society