AmericanChemicalSociety.com
Reports: UNI3 49494-UNI3: Electronic Tuning of Magnetic Exchange in Phenoxy-bridged Dinuclear Transition Metal Complexes
Vanessa P. McCaffrey, PhD, Albion College
Introduction
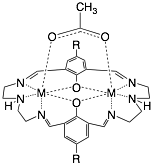
Phenoxy-bridged dinuclear transition metal complexes (see Figure 1) are ideal platforms to study fundamental magnetic exchange interactions. Their synthesis is straightforward and amenable to variety of structural modifications through substitution on the aryl rings or changing the identity of the linking amine bridge. Research in our lab during the previous funding period has been working towards elucidating how magnetic exchange is affected by altering the electron density of the phenoxy bridge through substitution on the aryl ring.
Figure 1. Generalized Schiff-base macrocycle.
Synthesis
During the past funding period, four undergraduates have been working on this project. They have synthesized five of the six desired dimanganese complexes where R is methyl, tert-butyl, bromo, chloro, or methoxy. All complexes have been characterized by IR and elemental analysis. The yields of the reaction have been very low and we have been working on changing solvents and other reaction conditions to increase the yield and purity of the complexes. In order to show that magnetostructural effects are not affecting the magnetic coupling, we have been working to grow crystals for X-ray crystallography of the dimanganese complexes.
The limiting step in the synthesis of the transition metal complexes is making enough of the 4-substituted-2,6-diformylphenol starting material. We have found that the traditional Duff reaction that makes these compounds also produces large amounts of monoformylated phenols. We have been exploring a variety of techniques in order to increase the ratio of dialdehyde to monoaldehyde in these reactions. To this end have developed new methodology for the synthesis of 4-substituted-2,6-diformylphenol compounds using microwave irradiation. We have increased the yields of the Duff reaction and decreased the reaction times by 90%. One student has shown an interest in computational chemistry and he has begun working on a project to model some interesting regiochemical preferences in the formylation of nitro-substituted phenols.
Magnetic Data
The magnetic susceptibility of the methyl, bromo and chloro have been measured and the value of J, the magnetic exchange interaction, for the four complexes is shown in Table 1.
Table 2: J values of substituted dimanganese complexes with Hammett sigma constants.
| s | sp+ | sp- | sJJá | J (cm-1) |
–Br | 0.23 | 0.15 | 0.25 | 0.23 | -3.4 |
–Cl | 0.23 | 0.11 | 0.19 | 0.22 | -2.8 |
–methyl | -0.17 | -0.31 | -0.17 | 0.15 | -2.9 |
–tButyl | -0.20 | -0.26 | -0.13 | 0.26 | -3.3 |
There does not yet seem to be a strong correlation with the standard Hammett sigma constants. We are currently looking at other physical properties of the phenoxy linkers to understand the variation within the J values as the substituent changes. It has been shown that in phenoxy radical, the spin density on the oxygen can be correlated with the HOMO-LUMO gap. We are working to apply this model to the magnetic data from our complexes. The difference in the HOMO-LUMO gaps that correspond to the complexes for which we have data is very small. The methoxy and trifluoromethyl HOMO-LUMO gaps are well separated from the halogen and alkyl substituents. We are working to purify the methoxy dimanganese complex for magnetic susceptibility measurements but the yield of purification is very low. We are also working on synthesizing the 4-trifluoromethyl-2,6-diformylphenol, but has only succeeded in making the monoformylated compound.
Copper Macrocycles
In addition to the manganese complexes, we have started work on the dicopper macrocycle analogues using the tert-butyl substituted starting material. Based on work in the literature, we have been changing the order of addition of reagents and varying solvent choice. We have recovered green solids that are show promise. We are working to fully characterize the complexes with UV/Vis, IR, X-ray crystallography and elemental analysis before analyzing the magnetic properties.
Integrating Research and Teaching
As a direct result of this project, I have developed an upper level laboratory experiment that we will be piloting in the Spring 2011 semester. Students will be performing the Duff formylation under traditional reflux conditions and then again using the microwave reactor. In addition to yields and product distribution (monoaldehyde:dialdehyde), they will be investigating the regiochemistry of the addition by using both para and meta substituted phenolic starting materials.
Funding Distribution
Funds from the first year of the UNI starter grant have been used to support four undergraduates during Summer 2009 and Summer 2010. Three additional students (including two underrepresented minorities) have worked on aspects of this project during the semester as directed studies. These students have given poster presentations at national ACS meetings, and oral presentations at a campus wide research symposium. One student who worked on this project was accepted into a highly competitive summer research program.
Copyright © American Chemical Society