AmericanChemicalSociety.com
Reports: AC7 47700-AC7: ROMP Healing Agent Development for Self-Healing Materials
Michael R. Kessler, Iowa State University
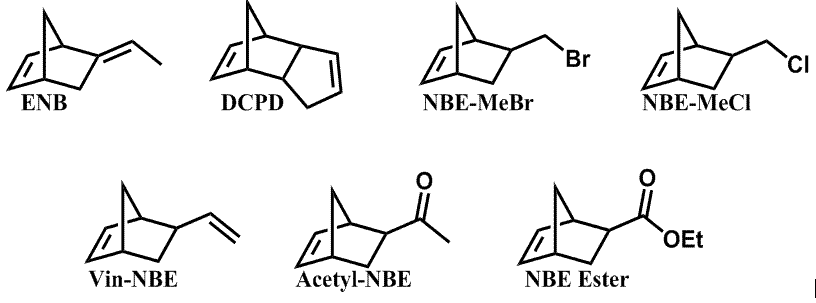
In the past decade, polymers and composites that can repair themselves with autonomy have been extensively studied in academia and received significant commercial interest [[1]]. Perhaps the most successful self-healing mechanism developed to date incorporates liquid healing agent-filled microcapsules and catalyst particles into a polymer matrix. Upon material fracture, microcapsules rupture, followed by flow of the liquid healing agent into the crack volume. When the healing agent contacts the catalyst particles it polymerizes and adheres the crack faces together [[2]]. The most well-studied, and probably most successful, healing agent/initiator combination used thus far in self-healing systems is dicyclopentadiene (DCPD) and Grubbs' catalyst, the former of which undergoes a reaction called ring-opening metathesis polymerization (ROMP) in the presence of the latter. This healing agent/catalyst system has been shown to only partially self-repair a fractured composite, and many factors have been identified elsewhere as responsible for this poor healing ability. However, very little work has been done to modify and optimize the DCPD/Grubbs' catalyst system to improve the self-healing capability. Hence, in this work we systematically develop novel healing agent/catalyst combinations (developed and screened in previous years' of this grant and shown in Figure 1) that satisfy the many unique requirements of a self-healing system. In the recent year of this ACS Petroleum Research Fund grant, research thrusts have focused on the development of a new rheokinetic technique designed to mimic the self-healing mechanism, and the application of this technique to study different healing agents.
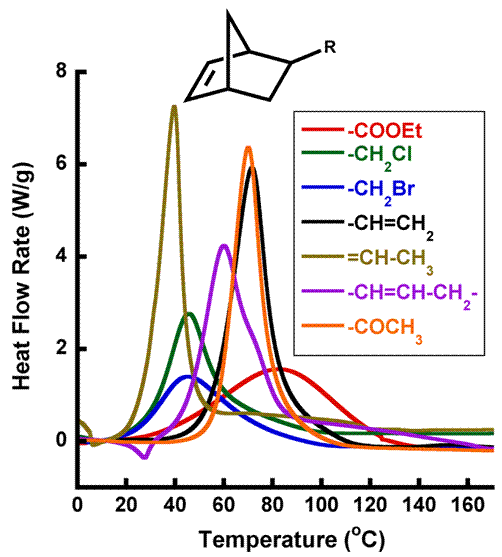
Fast polymerization kinetics is desired to self-heal in a reasonable time period, which initially led us to evaluate the bulk reaction kinetics of several of the healing agents with the Grubbs' catalyst (Figure 2). Polymerization kinetics was monitored by differential scanning calorimetry (DSC), which is an adequate measure of bulk reaction kinetics. However, self-healing is a complex mechanism that includes a number of different kinetic events (e.g. bulk polymerization kinetics, bulk catalyst dissolution kinetics, movement of dissolved catalyst throughout the monomer, traversing of monomer over the crack surface, etc.). For example, in a DSC curing experiment catalyst is pre-dissolved in monomer prior to the onset of significant of reaction, while in a self-healing environment the catalyst dissolution occurs concurrently with polymerization. Hence, while measuring the bulk reaction kinetics of various monomers via DSC does provide ample information vital to selecting ideal healing agents, an analytical technique designed to better yield information related to healing kinetics is desired.
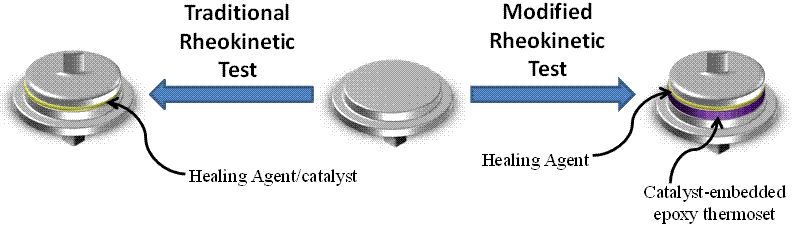
Building off of the PI's previous work [[3], [4]], we have developed a modified rheokinetic technique that is designed to mimic self-healing, and therefore yield useful information on the potential of different healing agents in self-healing materials. In a standard rheokinetic curing test, monomer is premixed with catalyst/curing agent outside of the rheometer instrument and the resulting solution is injected between two rheometer parallel plates (Figure 3, left arrow). In our modified technique, the bottom parallel plate is coated with a layer of epoxy thermosetting polymer containing embedded Grubbs' catalyst, and the surface of the coating is polished to reveal catalyst particles. Monomer (without pre-dissolved catalyst) is then injected between a top and modified bottom parallel plate (Figure 3, right arrow), in which the monomer is required to dissolve the embedded catalyst from the polymer surface as it polymerizes. This technique mimics the healing mechanism present in self-healing polymers while allowing for the rheological properties of the curing healing agent to be monitored.
It was determined that, of the seven different healing agents shown in Figure 1, only four (ENB, DCPD, NBE-MeBr, and NBE-MeCl) were able to polymerize in our modified rheokinetic technique at room temperature. The other three healing agents (Vin-NBE, Acetyl-NBE, and NBE Ester) did not show any polymerization, and were discarded from subsequent testing. The fact that Vin-NBE and Acetyl-NBE were unable to polymerize at room temperature is consistent with what would be expected from Figure 2; the onset temperature of both healing agents is well above room temperature. The onset temperature of the bulk polymerization of NBE Ester via DSC, however, is very close to room temperature. Hence, this healing agent would have been expected to show development of rheological properties over time. The reason for this unexpected failure of NBE Ester to react in a mimicked self-healing environment is likely a result of other kinetic parameters (e.g. catalyst dissolution kinetics) limiting the amount of catalyst available for the monomer to polymerize. The contradicting results between DSC and our modified rheological technique emphasize the importance of selecting an adequate analytical technique to study the effectiveness of healing agents in a self-healing material.
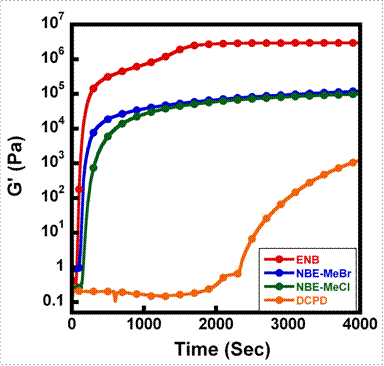
The developing dynamic mechanical properties of ENB, DCPD, NBE-MeBr and NBE-MeCl over time in our modified rheokinetic technique are shown in Figure 4. It is seen that ENB not only polymerizes faster than the other three monomers, but it also develops the highest maximum value of shear storage modulus. NBE-MeBr and NBE-MeCl also reach their maximum mechanical properties quickly, while DCPD still has not reached its maximum stiffness over the 2-hour timeframe of this experiment.
Figure 1. ROMP-active
healing agent library. Figure 2. Bulk
polymerization of various ROMP-active healing agents with Grubbs' catalyst at
heating rates of 20 K/min.
[Healing agent]:[catalyst] molar ratio for all
reactions is [3000]:[1]. Figure 3. Schematic of a
traditional rheokinetic experiment (left arrow) and a self-healing modified
rheokinetic experiment (right arrow), the latter of which was initially layered
with a Grubbs' catalyst-embedded epoxy coating. Figure 4. Rheokinetic
evaluation of ROMP-active healing agents at 25 °C using a modified, Grubbs'
catalyst/epoxy coated bottom parallel plate.
Copyright © American Chemical Society