AmericanChemicalSociety.com
Reports: ND10 48730-ND10: Engineering the Photosensitizer-Semiconductor Interface in Dye-Sensitized Solar Cells
Michael R. Detty, PhD, State University of New York at Buffalo
World energy demand is predicted to double by 2050 and triple by 2100. In the long term, solar energy is the most practical and abundant alternative energy source. The amount of solar energy reaching the earth's surface per hour (4.3 x 1020 J) exceeds the current annual world energy consumption (4.1 x 1020 J). However, solar energy currently provides just 0.1% of the world's electricity supply. Efficient solar energy conversion requires absorption of solar illumination followed by reduction and oxidation processes which compete with charge recombination. The light-to-electrical energy conversion mechanism of dye-sensitized solar cells (DSSCs) using nanocrystalline TiO2 involves electron injection by the photoexcited sensitizer followed by electron transport through the TiO2 film and the external circuit. The oxidized sensitizer is reduced by an electron donor in the electrolyte, which is subsequently re-reduced at the counter electrode. In addition, charge separation at the semiconductor-photosensitizer interface leads to long-lived charge-separated states, enabling productive redox processes to compete with electron-hole recombination.
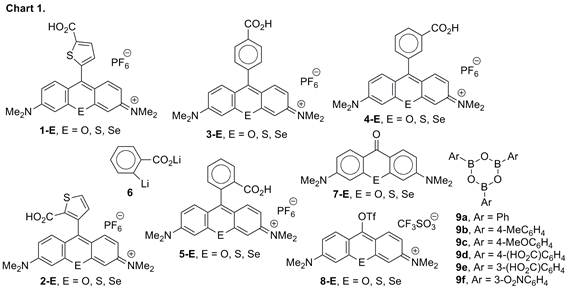
We have been examining chalcogenorhodamine dyes that form aggregates on nanocrystalline TiO2. These aggregated organic photosensitizers broaden the absorbance bands to both the shorter-wavelength side (H-aggregates) and the longer-wavelength side (J-aggregates) of the unaggregated dye absorbance maximum. Our preliminary data indicated incident-photon-to-current efficiency (IPCE) in several DSSCs based on H-aggregated dyes 1-E (E = O, S, Se, Chart 1) was much higher than in DSSCs based on amorphous, non-aggregated dyes 2-E (E = O< S, Se, Chart 1)). The carboxylic acid functionality in dyes 1-E and 2-E is necessary to bind the dyes to the TiO2 surface.
In
order to examine the series of dyes 3-E,
4-E, and 5-E (Chart 1) with 9-(carboxyphenyl) substituents for their ability to form H-
and/or J-aggregates, it was necessary to devise synthetic schemes that would
allow their synthesis. Dyes 5-E can be prepared by the addition of
lithium 2-lithiobenzoate (6) to the
corresponding chalcogenoxanthone 7-E, followed by acid-induced dehydration. This approach does not work for the synthesis
of dyes 3-E and 4-E since the corresponding 4-lithio and 3-lithiobenzoates (or the
corresponding Grignard reagents), respectively, are thermodynamically unstable
and quickly isomerize to 6. We circumvented this problem using a modified
Suzuki-coupling procedure. The chalcogenoxanthones 7-E
reversibly form the triflates
8-E under anhydrous conditions. While the boronic
acids hydrolyze the triflates back to the starting xanthones, the triflates 8-E were stable in the presence of arylboroxins 9
and the addition of sodium carbonate and 10 mol-% of
PdCl2(PPh3)2 gave the corresponding
9-arylchalcogenorhodamines (including 3-E
and 4-E) in excellent yield. The
series of dyes 3-E, 4-E, and 5-E (Chart 1) behaved
quite similarly to the 9-thiophene carboxylic acid derivatives 1-E and 2-E. Dyes 3-E and 4-E formed H-aggregates on nanocrystalline
TiO2 while dyes 5-E (“traditional”
rhodamine derivatives) did not form H-aggregates and
gave an amorphous coating of dye on the TiO2. In this series of dyes, IPCE values for the unaggregated dyes 5-E
on TiO2 were higher than IPCE values for the H-aggregated dyes 3-E and 4-E in work done in collaboration with Prof. David Watson at the
University at Buffalo. However, IPCE
values for dyes 5-E on TiO2
were smaller than those of dyes 1-E
on TiO2 suggesting that dyes 5-E
might be less efficient at converting sunlight into electricity However,
dyes 5-E are among the most
efficient photosensitizers ever examined for the evolution of hydrogen from
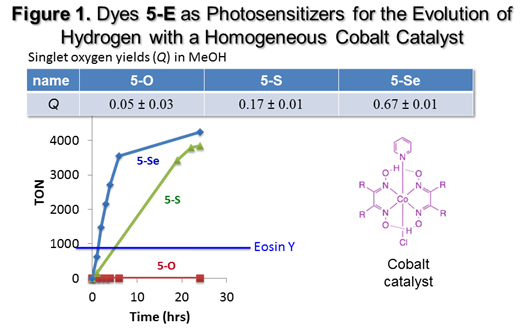
water in either a homogeneous system (Figure
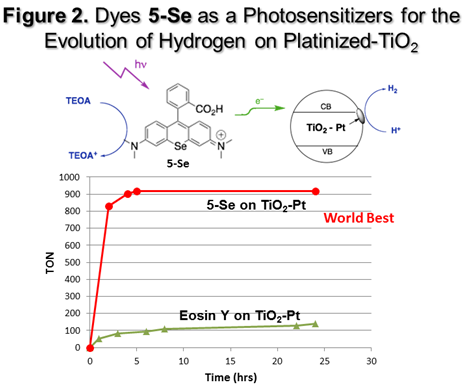
1) or in a heterogeneous system (Figure
2). The following work was done in
collaboration with Prof. Rich Eisenberg and Dr. Theresa McCormick at the
University of Rochester. In the presence of triethanol
amine (TEOA) as a sacrificial electron donor and the cobalt catalyst shown in
Figure 1, dye 5-Se gave nearly 5000
turnovers for the production of hydrogen in 24 h compared to 3500 turnovers for
dye 5-S, and no hydrogen evolution
with dye 5-O. The production of hydrogen tracks the triplet
yield [as measured by the singlet oxygen yield (Q)], which suggests that electron transfer is more competitive from
the longer-lived triplet state.. Further modifications of the system have
given turnover numbers of >10,000 after 24 h. Both 5-Se
and 5-S are much more efficient than
Eosin Y, which was the previous “best” photosensitizer for this system. On
platinized TiO2 using TEOA as a sacrificial electron donor, dye 5-Se was far more efficient as a
photosensitizer on platinized TiO2 than Eosin Y, which again was the
previous world “best” photosensitizer for hydrogen evolution on platinized TiO2. As shown in Figure 2, dye 5-Se gave
over 900 turnovers in the first 5 hours of reaction while Eosin Y gave
approximately 100 turnovers. Dyes 5-S and 5-Se were far more efficient in either the homogeneous and
heterogeneous systems than the other carboxylic acid containing dyes 1-E, 2-E, 3-E, or 4-E. During
the next reporting period, we shall prepare other chalcogenorhodamine
derivatives with carboxylic acid functionality on the amino substituents in
order to examine J-aggregation. We shall
also examine the oxidized photosensitizer on TiO2 by transient
techniques in order to examine lifetimes and recombination rates. We shall also examine ways to optimize the
evolution of hydrogen both in heterogeneous and homogeneous systems. In particular, we shall examine the lifetimes
of the reduced photosensitizer as a means to prolong the period of hydrogen
evolution. The
PRF support has allowed the PI to become involved with solar fuels and solar
electricity by providing support for a core group of students. The graduate students on this project have
benefited not only from the financial support, but also from the breadth of
science involved in this project: synthesis, catalysis, photophysics,
materials science.
He undergraduate student participating in this project is applying to
various programs for graduate studies in chemistry. (He originally was
interested in chemical engineering!)
Copyright © American Chemical Society