AmericanChemicalSociety.com
Reports: AC3 47426-AC3: Discovery of Molecular Oxidation Catalysts using Metallo-Enzyme Mimicry and Combinatorial Chemistry
Stephen B. Colbran, University of New South Wales
Aims:
The aims of this project were:
(1) To develop routes to new polytopic ligands and/or ligand precursors capable of forming multi-metallic complexes combining organometallic N-heterocyclic carbene (NHC) centres and one or more 'classical' metal sites.
(2) To find methods to selectively form multimetallic complexes of the afore-mentioned ligands or ligand precursors.
(3) To use these selective methods to prepare libraries of multimetallic complexes that combine organometallic – organic substrate activating – centres with classical – dioxygen/peroxide activating – centres.
(4) To assay these libraries for efficient catalysis of selected oxidations of organic substrates.
Personnel:
A/Prof. S.B. Colbran and Prof. D.B. Hibbert were the principal investigators. SBC was involved in all aspects of the project. DBH is a chemometrician and he was to design efficient methodologies to screen of the libraries of complexes for oxidative catalyses. This proved unnecessary as only a small library of complexes could be obtained for catalysis assays.
ACS-PRF funds were used to support three researchers that performed all of the laboratory work: Dr Sang-Tae Lee was employed as a part-time research assistant working on the synthesis throughout the project (starting July 2008). Most of the results summarised below are from his work. Mr Alex McSkimming, a third year BSc student, was part funded as a summer scholar from the ACS-PRF grant, and Mr Matthew Peterson, another third year BSc student, was funded part time from the ACS-PRF grant to prepare the mono-metal equivalents of each metal centre in the multi-metallic species, and to assay these and mixtures of these for oxidation catalyses. A fourth researcher, PhD student Mr Ivan Taylor, performed DFT calculations that were inspired, but not funded, by this project.
Results:
The results obtained from this project were:
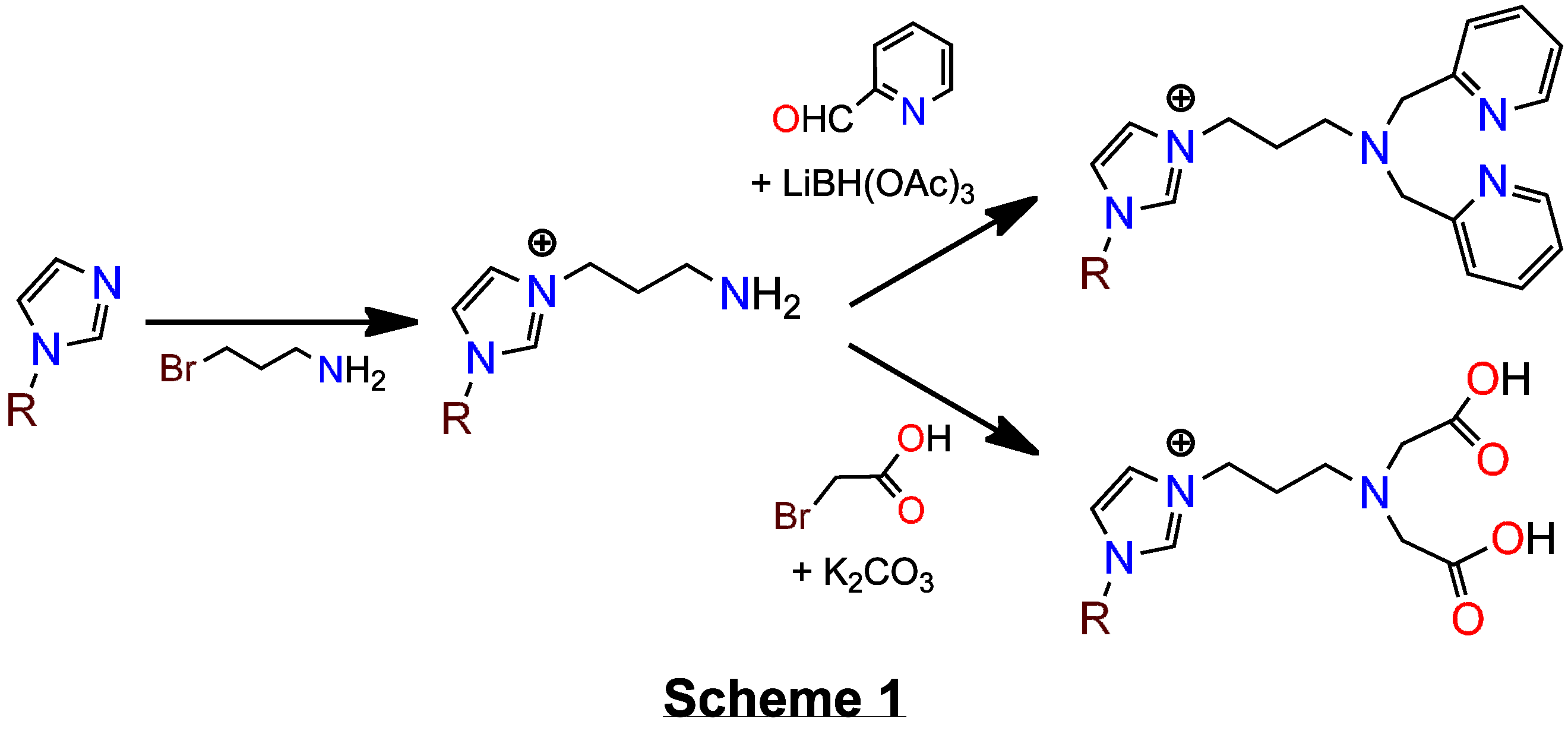
(1) After several unfruitful mis-directions, a new route to imidazolium salt precursors to NHC-ligands that are tethered to 'classical' donor groups was discovered. The route is simple and has great versatility. We find that treatment of any imidazole with 3-bromopropan-1-amine affords the amine-tethered imidazolium salts, which are readily isolated as the PF6– salts upon metathesis with NH4PF6 in methanol–water mixtures, see Scheme 1. The amino-tether can be then elaborated by standard methods to build-up the classical donor groups, e.g. reductive amination with 2-carboxaldehyde pyridine afforded a pendant bis(2-pyridylmethyl)amine (bpa) donor and alkylation with 2-bromoacetic acid gave a pendant amino(diacetic acid) donor, Scheme 1.
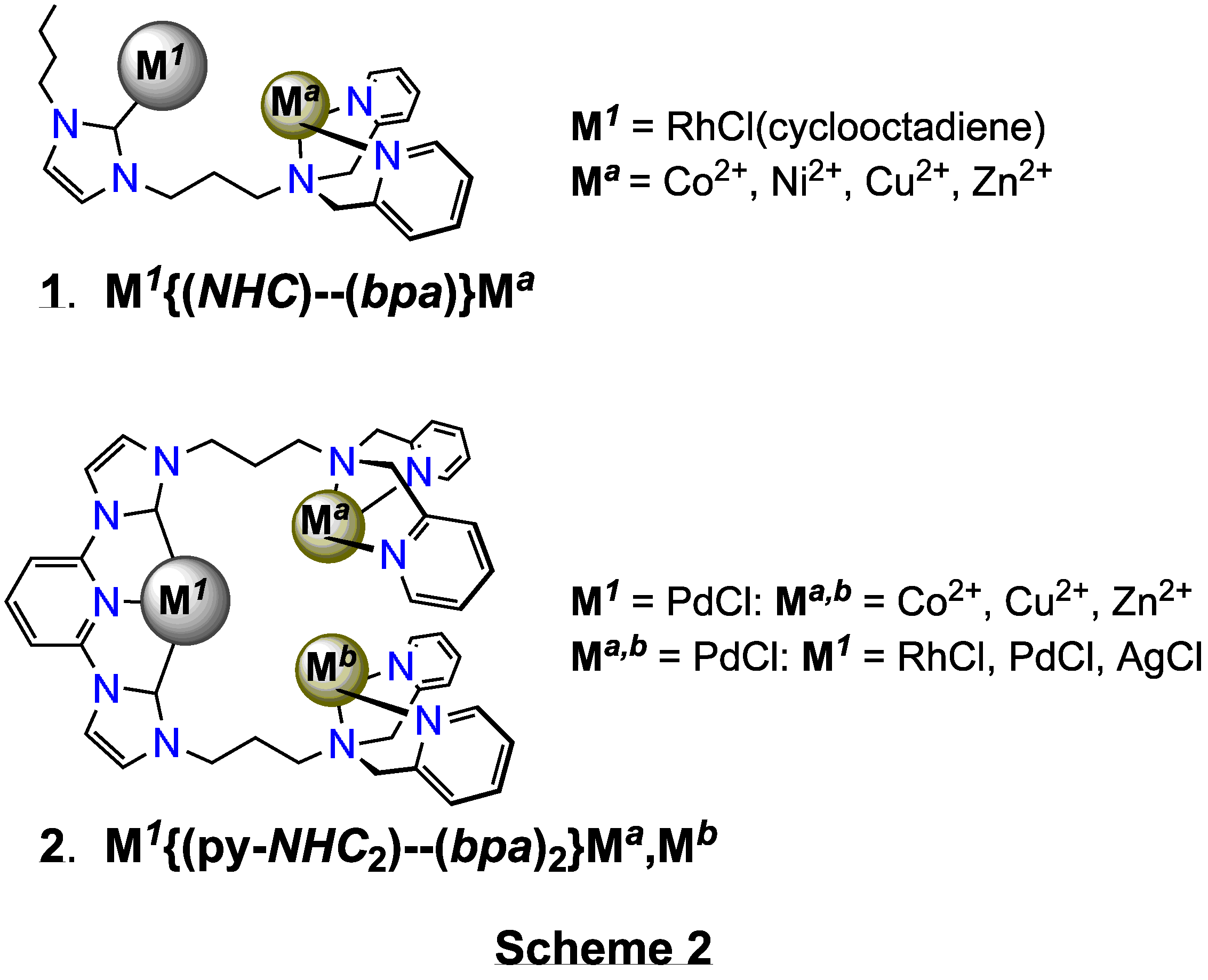
(2) Selective
metalation of the various metal centres in the polytopic ligands was not as straightforward
as we thought it would be, and always proved to be problematic. For example, complex
(1) in Scheme 2 was limited to a RhCl(cyclooctadienyl)
organometallic centre because, despite many attempts, selective routes to other
organometallic centres, e.g. of Ru or Pd, were not found. Palladium(II)
precursors, for example, always bound at both the NHC
and the classical bpa donor domains. While not the
targets of this work, such species are new and of interest in
themselves.
(3) Tying-up
the 'classical' domain with palladium(II) was relatively easy and facilitated
subsequent selective metalation of the organometallic domain. For example,
'strong' coordination of the two bpa domains in (2)
with, e.g., 'PdCl' (introduced as PdCl2(PhCN)2)
allowed selective metalation of the 2,6-bis(imidazolium)pyridine domain to
afford trimeric systems with{bis(NHC)-pyridine}RhCl, {bis(NHC)-pyridine}PdCl
and {bis(NHC)-pyridine}AgCl organometallic centres, Scheme 2. (4) Monomeric
analogues of each of the metal centres in dimers (1)
and trimers (2) were prepared. (5) The
dimers (1),
trimers (2), and the monomeric analogues of each of the metal
centres in these, were serially assayed (using GC-MS analysis) as catalysts for
the oxidation of styrene by
hydrogen peroxide. Both epoxidation and oxidation to benzaldehyde and benzoic
acid were observed. Unfortunately, none of the complexes showed high activity
or selectivity for a single oxidation product. Disappointingly, the oligomers
were, at best, only as active as a mixture of the component metal centres. (6) DFT
calculations were performed to investigate the ease of formation and the
oxygenation/H-atom abstraction activities of metal(IV)-oxo (MIV=O: M = Ti–Cu) species
— akin to the putative intermediates formed at the "classical"
centres in the target oxygenation reactions. The results revealed that the activities
should increase with increasing radical character for the oxo ligand —
i.e. as the species takes on more MIII–O¥
character. The MIII–O¥
character, as expected, scales with atomic number (increasing effective nuclear
charge) and, therefore, the NiIV=O and CuIV=O species are
predicted to be most active in oxygenation reactions.
Copyright © American Chemical Society