AmericanChemicalSociety.com
Reports: AC7 47951-AC7: Replacing Polyvinyl Chloride with Novel Thermoplastics Derived from Natural Gas
Stephen A. Miller, University of Florida
Researcher story
The primary research objective of this proposal is to synthesize novel polyoxymethylene copolymers—derived from natural gas feedstocks—having thermomechanical properties suitable for replacing the common thermoplastic polyvinyl chloride (PVC). The main significance of these research endeavors is linked to the growing recognition that PVC is environmentally problematic during all stages of its chemical lifecycle. The synthesis and identification of functional PVC substitutes will positively impact the polymer industry. Polyoxymethylene (POM) is already a commercial material with many excellent properties, but complex copolymers based on POM are rare and poorly understood. The organic feedstock for POM is methanol, which currently is derived from natural gas, a fossil fuel with a projected availability longer than that of petroleum
We have performed a number of enlightening computational studies. The ability of an alkyl branch to depress the melting temperature in a polyoxymethylene chain is measurably less than that in a polyethylene chain. The factors that inhibit the alkyl-branch plasticization of polyoxymethylene were considered by computational assessment of a series of model compounds at various levels of theory: DFT B3LYP 6-31+G*, DFT B3LYP 6-311++G**, MP2 cc-pVTZ, T1, and G3(MP2).
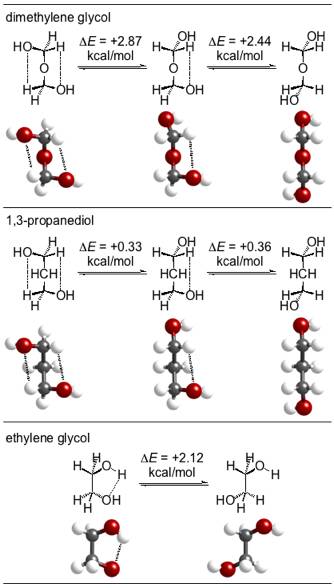
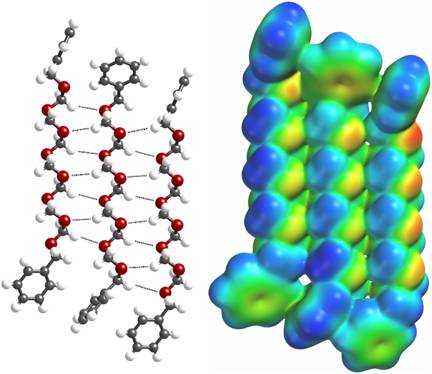
An important model for the polyoxymethylene chain can be found in 1,17-diphenyl-2,4,6,8,10,12,14,16-octaoxaheptadecane (Ph(CH2O)8CH2Ph), which contains eight oxymethylene units and has previously been structurally characterized by X-ray crystallography (J. W. Bats, C. Miculka, C. R. Noe, Acta Cryst. 2007, C63, o190-o192.) We have analyzed the packing structure of this compound in search of intermolecular chain-chain interactions. Consider the lattice plane depicted in Figure 1. These chains, indeed, appear to have significant interaction between the delta+ of the acetal hydrogens and the delta– of the oxygens. The electrostatic potential map reveals significant electron density between the chains. Intramolecular interactions—characterized as acetal CH...O hydrogen bonds—are surprisingly strong and likely encourage conformational regularity in the vicinity of the alkyl branches, allowing maintenance of the intermolecular chain-chain interactions. These interactions explain the known all-gauche conformation of polyoxymethylene.
Figure
1 The intramolecular
interactions were modeled computationally with more tractable small
molecules. The acetal CH...O hydrogen bonds in dimethylene glycol average to 2.65 kcal/mol while the
non-acetal CH...O interactions in 1,3-propanediol are much weaker with an
average of 0.34 kcal/mol (G3(MP2)). The related,
classical OH...O hydrogen bond in ethylene glycol is found to be worth 2.12
kcal/mol (Figure 2). To describe
this energetic ordering, an additional stabilizing anomeric
effect is invoked for dimethylene glycol, a model for
polyoxymethylene. Figure
2 The strong inter- and intramolecular
forces suggest that significantly plasticized polyoxymethylene
copolymers will require rather high comonomer
compositions. For this and other reasons we continue our pursuit of more
effective and economical approaches to polyoxymethylene
plasticization. Figure
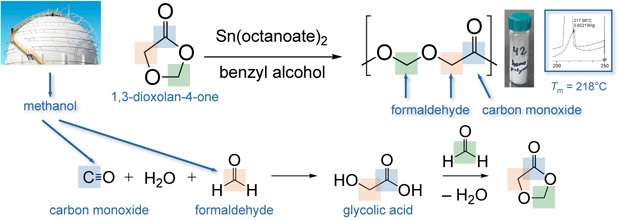
3 depicts strategy that exploits C1 feedstocks
and achieves a greater effective comonomer content in
polyoxymethylene chains. The synthesis of
1,3-dioxolan-4-one is achieved by the condensation of glycolic acid with
formaldehyde. Since glycolic acid can be obtained via the carbonylation
of formaldehyde, this 3-carbon heterocycle can be made
exclusively from C1 feedstocks. Upon ring-opening
polymerization, the obtained polymer is a regioregular
polyesteracetal, which contains a novel juxtaposition
of functional groups in polymer chemistry. Poly(1,3-dioxolane-4-one)
is, fundamentally, the regular copolymer made from two parts formaldehyde and
one part carbon monoxide. This is a new, semicrystalline
thermoplastic that has a melting temperature near 218 degrees C. The
monomer 1,3-dioxolane-4-one can also be copolymerized with a variety of lactones.
This is a viable strategy for modulating the physical properties and the
degradation pathways of polylactide and polycaprolactone. Figure
3 Computational studies (G3(MP2))
imply that the ring-strain of this five-membered 1,3-dioxolane-4-one (deltaH = –6.7
kcal/lmol) is similar to that of gamma-butyrolactone
(deltaH =
–7.6 kcal/mol), which has unfavorable ring-opening polymerization
thermodynamics (Figure 4).
Nonetheless, opening of 1,3-dioxolan-4-one engages two specific anomeric interactions with oxygen lone pair donations into
the sigma* of an adjacent C–O bond (worth 2.8 kcal/mol) and an adjacent
C–C(O) bond (worth 1.5 kcal/mol). These
presumably restrict polymer conformations, contribute to crystallization
polymerization, and allow for overall favorable polymerization thermodynamics. Figure
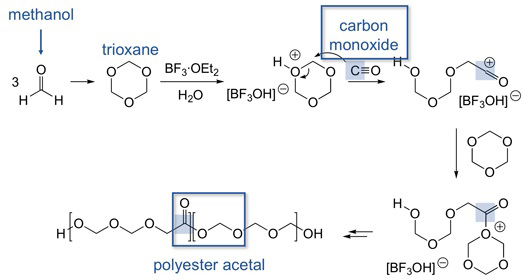
4 A method for modulating the properties of polyoxymethylene with a very inexpensive and practical comonomer is the cationic copolymerization of trioxane and carbon monoxide. Figure 5 depicts our strategy for this copolymerization. Reaction of the cationic chain end with
carbon monoxide results in an acylium ion that can
continue to react with trioxane. At that point in the polymer chain, an
ester functional group will result. Figure
5 Although carbon monoxide can be a reluctant nucleophile toward cations, high
pressures of CO (800 psi) and high temperatures
(100°C) have allowed significant incorporation, presumably resulting in the
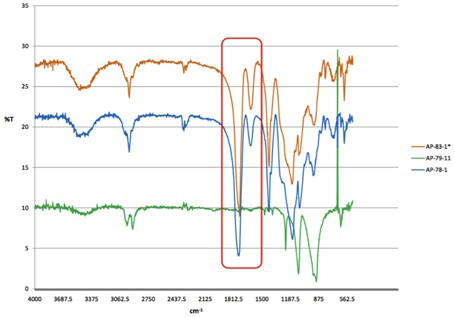
targeted ester functionality. Figure 6 confirms the presence of the
ester functional group in the polymeric product (IR stretch at 1780 cm-1
vs no peak in the trioxane homopolymer) and we are now focused on making a series of
such polymers and characterizing them fully. Figure
6
Copyright © American Chemical Society