AmericanChemicalSociety.com
Reports: AC3 45990-AC3: New Silanimine and NSi-H-M Agostic Complexes for Coupling with Petroleum Derived Products
Georgii I. Nikonov, PhD, Brock University
In the final grant period the following results were accomplished:
1) Given our original goal to study the reactivity of tripodal/imido complexes towards silanes and our previous mechanistic studies on reactions of Cp(ArN=)Mo(H)(PMe3) (1) with silanes and carbonyls, we turned to the isolobal complex Tp(ArN=)Mo(H)(PMe3) (2, Tp= tris(pyrazolyl)borate). The structure of 2 was established by spectroscopic methods and X-ray diffraction. Unlike its Cp-analogue 1, which exchanges the PMe3 ligand with free phosphine by an associative mechanism, kinetic measurements for 2 revealed a dissociative mechanism which involves dissociation of one of the three legs of the Tp-ligand to free up a coordination site. 2 was found to catalyze hydrosilylation of carbonyls. Unfortunately, like the previously studied tris(carbene) tripods, complex 2 does not react with silanes. Instead, mechanistic studies of catalytic hydrosilylation showed that 2 adds benzaldehyde to furnish the alkoxy derivative (Tp)(ArN)Mo(OCH2Ph)(PMe3) (3). But unlike the similar reaction of 1, this process is dissociatively activated (DS≠ = 45.3 ± 53.45 J/(mol*K)). Complex 3 reacts further with PhSiH3 to regenerate the hydride, thus closing the catalytic cycle.
2) In order to understand the catalytic nitrile hydrosilylation by complex (ArN=)(Me3P)3Mo(H)(Cl) (4) reported in the previous grant period, we studied reactions of 4 with nitriles and PhSiH3. The new vinylidenamido complex (ArN=)(Me3P)2Mo(Cl)(h1-N=CHPh) (5) was obtained when 4 reacts with benzonitrile. Complex 5 was studied by NMR and X-ray diffraction. It reacts with PhSiH3 to regenerate the starting hydride but this reaction requires longer times and elevated temperatures and is the slowest step in the catalytic process. We also discovered that 4 reacts with excess silane to give the new complex (ArN=)(Me3P)3Mo(SiH2Ph)(Cl) (5) whose reactivity is still the subject of ongoing research.
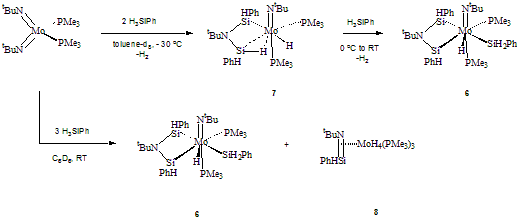
3) Reaction of the bis(imide) (tBuN=)2Mo(PMe3)2 with excess PhSiH3 results in the new complex {h2- tBuN(PhHSi)2}Mo(PMe3)2(H)(SiH2Ph) (6, Scheme 1), which is a rare example of triple silane addition to a metal complex. This result also highlights our notion that the imido ligand can be coupled with silanes and eventually detached from the metal, which may be a step in the proposed tricomponent coupling of an amine, silane, and unsaturated organic substrate. We have reliably established the structure of 6 by NMR spectroscopy and X-ray diffraction. Detailed investigation of its reactivity will be the subject of future work. When the formation is followed by low-temperature NMR, clean formation of the double silane addition intermediate (h3-(tBuN)(PhHSi)(SiHPh-H…)Mo(PMe3)2(H) (7) was seen. Complex 7 is SiH-M agostic on the basic of a 29Si NMR study. Surprisingly, when (tBuN=)2Mo(PMe3)2 reacts with three equivalents of PhSiH3, a 1:1 mixture of 6 and the silanimine (h2- tBuN=SiHPh)Mo(PMe3)3(H)4 (8) forms. The latter product was characterized by NMR spectroscopy. Unfortunately, its instability precluded a preparative scale reaction.
Scheme
1.
Overall, this PRF grant generated a
wealth of results and allowed me to train three students in synthetic
organometallic chemistry, catalysis, and mechanistic studies. The main
highlights of this research are the discovery of new Si-C and Si-N coupling and
Si-N bond cleavage reactions as well as the discovery of new mechanistic
pathways in the hydrosilylation of carbonyls. In some cases we proved the
intermediacy of silanimine and SiH…M agostic complexes.
Copyright © American Chemical Society

