AmericanChemicalSociety.com
Reports: G7 48488-G7: Investigation of the Relationships Between the Morphology and Transport Properties of Sulfonated Block Copolymers Containing Glassy Hydrophobic Blocks
Kevin A. Cavicchi, University of Akron
Overview: Ion-exchange membranes are important in a number of applications, such as fuel cells and water purification. Self-assembled nanostructures, such as those obtained with neutral-polyelectrolyte block copolymers for example, are ideal for fabricating membranes. Here the concentration of the ionic groups provides good transport properties, while the confinement of ionic domains limits membrane swelling improving the membrane selectivity. The focus of this research has been on the synthesis and fabrication of new nanostructured ion-exchange membranes. Methods have been developed which allow for ease and flexibility in both the synthesis of polymers and the fabrication of membranes.
Research Highlights: In the first year of this project the major accomplishments were first, the preparation of poly(trithiocarbonate) reversible addition fragmentation chain transfer (RAFT) agents for the polymerization of (AB)n multiblock copolymers and second, the direct polymerization of neutral-sulfonated AB block copolymers through the synthesis of hydrophobic alkylammonium styrene sulfonate monomers. This past year research concentrated on the synthesis and characterization of long-chain poly(dimethyl n-alkylammonium styrene sulfonate) polymers. It was found that these polymers could act as organogelators for the preparation of thermoreversible gels in organic solvents. The gelation behavior was dependent on the structure of the polymer where gels were stabilized at higher temperature and in solvents of increasing polarity by increasing the backbone degree of polymerization and/or the length of the alkyl side-chains. As an example the behavior of poly(dimethyl octadecylammonium styrene sulfonate) (PSS-NC18) in benzene is presented below.
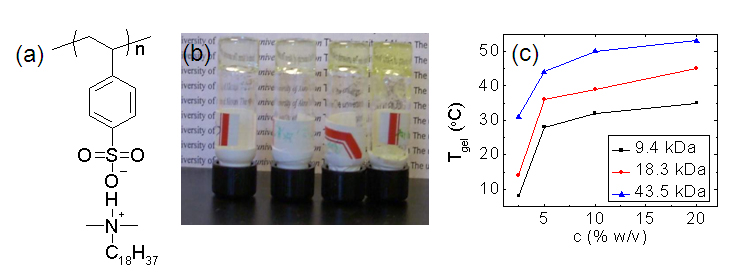
Three PSS-NC18 polymers with molecular weights of 9.4, 18.3, and 43.5 kDa and polydispersity indices of 1.3-1.4 were prepared by RAFT polymerization. The chemical structure of PSS-NC18 is shown in Figure 1a. At room temperature these polymers were insoluble in benzene. However, they could be dissolved at elevated temperatures and upon cooling self-supporting gels were formed that did not flow when inverted as shown in Figure 1b. Figure 1c shows measurements of Tgel vs. concentration determined by the temperature at which the gels first flowed when inverted during heating.
Figure 1. (a) Structure of
PSS-NC18, (b) 18.3 kDa
PSS-NC18/benzene gels at 2.5, 5, 10, and 20 wt/vol% polymer (L to R), (c) Tgel
vs concentration for PSS-NC18 in benzene . The morphology of the gels was
investigated by scanning electron microscopy (SEM) on freeze-dried xerogels.
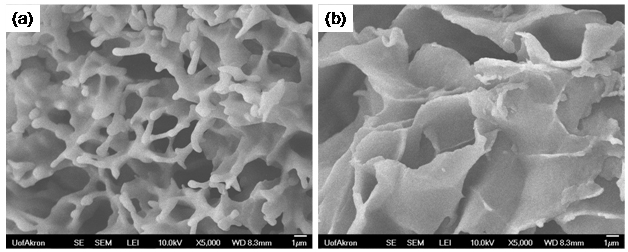
Figure 2 shows two freeze dried xerogels obtained from 10 wt/vol% polymer in
benzene using either the 18.3 kDa or 43.5 kDa PSS-NC18. Both samples had
network-like morphologies, however there is a clear molecular weight dependence
of the morphology where the low molecular weight polymer contains
interconnected cylindrical struts, while the high molecular polymer has a
sheet-like morphology. These network morphologies are believed to be driven by
the amphiphilic nature of the PSS-NC18 polymers. An analogy can be made to
small molecule surfactants where at high temperature the polymer is soluble in
solvent, however at lower temperature reverse-micellar aggregates are formed to
shield the ionic groups from the non-polar organic solvent. Figure 2. Xerogels of (a)
18.3 kDa and (b) 43.5 PSS-NC18 prepared from 10 wt/vol% PSS-NC18/benzene gels. Significance of the
Research: A
new organogelator system has been developed and is useful as an additive to
modify the viscoelastic properties of solvents. In addition, the network
morphology of these organogelators could template new porous membrane
materials. Future work will explore the gelation and polymerization of organic
monomers, where after polymerization pores or polyelectrolyte threaded pores
will be generated by removal of the organogelator or solely the surfactant
side-chains, respectively. Success of this approach would provide a new method
to fabricate ion-exchange membranes with concentrated percolating ionic
domains. This approach has the advantage of potentially decoupling the pore
morphology (sheets, cylindrical or spheres) from the ion concentration. Therefore,
confined ionic channels would be obtained at much higher polyelectrolyte
loading levels than in comparable block copolymer systems. In neutral-ionic
block copolymers high polyelectrolyte block volume fractions invariably lead to
an unconfined ionic matrix, which exhibit poor resistance to swelling and low
solvent selectivity. Impact of PRF
Funding: These
funds from the PRF have been instrumental in supporting the PI's research group
at the start of his career. One graduate student (Yuqing Liu) has been
supported for two years. Travel support from this project allowed him to
present a poster and an oral presentation at the 2009 and 2010 spring National
American Chemical Society meetings, respectively. Two papers have been
published from this work in peer-review journals.
Copyright © American Chemical Society