AmericanChemicalSociety.com
Reports: UNI5 49523-UNI5: Identification of Photocatalytically Active Surface Sites on Layered Transition Metal Sulfides
Tykhon Zubkov, PhD, Ball State University
MoS2 and WS2 are layered sulfides that can exhibit photocatalytic properties when dispersed on a nanometer scale.1-4 Hypothesis: the edge sites on the crystallographic sheets play dominant role in the photochemistry as opposed to basal planes.
The progress involved several directions:
1. Construction of the high-vacuum setup for transmission infrared spectroscopy of surface species has been nearly completed using commercial and custom-made parts. The goal is to study how organic molecules adsorb on specific surface sites on MoS2 and WS2 and to monitor their chemical transformations induced by photoexcitation of charge carriers in the sulfides. The vacuum cell for IR spectroscopic measurements under UV-visible irradiation is equipped with pressure gauges, evacuated by pumps to a base pressure of 1x10-7 Torr, and connected to a gas delivery line and to the quadrupole mass-spectrometer in close proximity. A tungsten grid inside the cell supports a powdered sample and provides cooling and heating. A similar high-vacuum station will share the existing gas delivery line. The two systems are to share Bruker Tensor 27 FTIR spectrometer alternatively using a specifically designed optical bench with a system of mirrors. (Funding: ACS PRF and Lilly V start-up grant.)
2. Surfactant-free synthesis of the MoS2 and WS2 nanoparticles and tests of their photocatalytic activity.
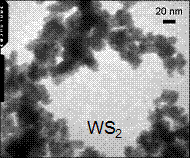
Any surfactants used in the nanoparticle synthesis can be difficult to remove, which would adversely affect subsequent surface chemistry experiments. Synthesis proposed by Duphil et al. is surfactant-free,5 however, the optical and photocatalytic properties of the resulting particles were not reported. This method was modified for our study. MoS2 and WS2 nanoparticles were synthesized by decomposing the metal hexacarbonyl in the presence of sulfur dissolved in decalin at 140°C. The particles were purified by washing and centrifugation cycles. As opposed to the black color of bulk MoS2 and WS2, dried nanoparticles are reddish in color (Fig. 1), signifying the onset of the quantum confinement in this size range. In agreement with this, the nanoparticles exhibited a size-dependent shift in their threshold UV-visible absorption in sedimentation experiments. A significant fraction of the particles was ≤ 15 nm in diameter as verified by TEM (Fig. 1).
Fig. 1. (Left) Dried MoS2 and WS2 nanoparticles. (Right) TEM of the MoS2 and WS2 nanoparticles.
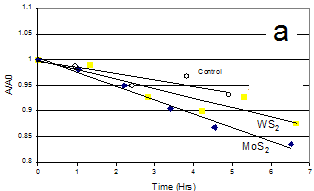
Photocatalytic tests were performed using 300W Xe arc discharge lamp with long-pass cut-off filters. MoS2 and WS2 nanoparticles catalyzed the photodegradation of acetone in water under visible light of ³400nm wavelength (Fig. 2a). To our knowledge, this is the first study reporting the photocatalytic properties of the unsupported WS2 nanoparticles. The above sulfide nanoparticles were highly hydrophobic and remained adhered to the bottom of the reactor rather than being uniformly dispersed in the aqueous solution. This lowered the reaction rates and makes it difficult to reproduce the active surface area between experiments.
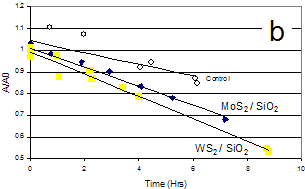
The challenge was addressed by “solubilizing” the photocatalyst in water by depositing it onto a highly dispersed hydrophilic substrate. The sulfide nanoparticles were synthesized in the presence of dispersed SiO2 or TiO2 to produce 1%wt supported metal sulfide nanoparticles. These materials could be uniformly distributed in aqueous solutions to maximize the photocatalytic efficiency. Silica- and titania-supported sulfides significantly catalyzed the photodegradation of methylene blue under ³ 435nm irradiation (Fig. 2b). Thus we have demonstrated that photocatalytically active sulfide nanoparticles can be synthesized surfactant-free.
Fig. 2. (a) Photodegradation of acetone in water by MoS2 and WS2 nanoparticles under visible light. (b) Photo-degradation of methylene blue in water by MoS2/SiO2 and WS2/SiO2 under visible light.
3. Size fractionation of the MoS2 nanoparticles (ongoing).
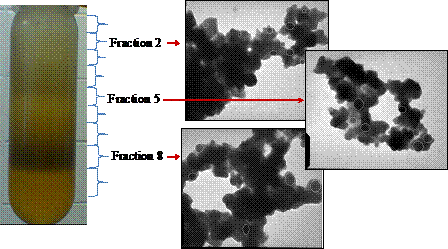
Photocatalytic ability of MoS2 is strongly affected by the magnitude of its band gap, which in its turn is strongly size-dependent. The above synthesis produces polydisperse materials in the 5-30 nm range. Yet, systematic studies would require narrower size-selected fractions. Methods reported in literature for various materials include density gradient centrifugation6 or size-exclusion chromatography.7,8 In this study, we attempted size fractionation of the synthesized MoS2 nanoparticles using several methods. Sedimentation centrifugation in cyclohexane and density gradient centrifugation in cyclohexane-bromoform gradient resulted in some crude fractionation (Fig. 3). However, the fractions are very polydisperse with the particle size varying by a factor of 2-3. Currently, we are attempting to use size exclusion chromatography to achieve better separation with a narrow size distribution.
Fig. 3. Crude size-fractionation of the MoS2 nanoparticles in the cyclohexane-bromoform gradient. Centrifuge tube shows a typical distribution of MoS2 after centrifugation. TEM images span 730x880 nm. Selected particles are marked for clarity with contours.
4. Exfoliation of the MoS2 and WS2 Atomic Layers.
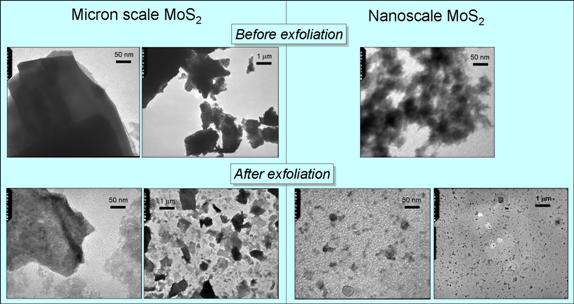
To elucidate where the photo-produced electrons and holes in layered sulfides exit and transfer onto adsorbates, we would greatly benefit from having quazi-two-dimensional nanoparticles only one layer thick. MoS2 or WS2 sheets of one or several atomic layers can be exfoliated using intercalation with n-butyllithium followed by reaction with water. Previous reports dealt with exfoliated micron-sized sheets.9,10 We explored the applicability of this method for the production of nanosized sheets from nanoparticles. Synthesized MoS2 and WS2 nanoparticles were exfoliated. Nanosized sheets of MoS2 and WS2 were produced as verified by TEM (Fig. 4).
Fig. 4. TEM of commercially available micron-sized MoS2 particles and synthesized nanoparticles before and after exfoliation.
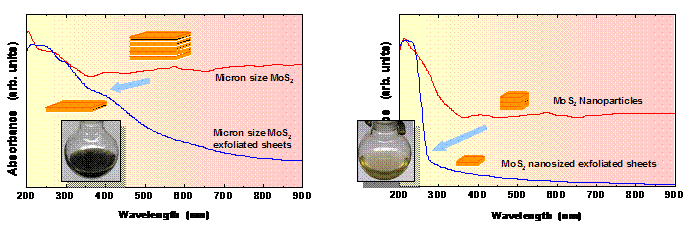
Due to strong quantum confinement in the atomically thin nanosheets,
optical absorption is strongly shifted from visible to the ultraviolet
region. These nanosheets are almost
colorless or a pale yellow compared to the brown color of micron-sized sheets (Fig.
5). The preparation of size-selected
atomically thin MoS2 and WS2 with systematically varying
edge-to-plane ratio will help us correlate the photocatalytic activity to
particular surface sites. Funding: ACS PRF and Fig. 5. UV-visible absorption spectra of micron-sized MoS2
particles and synthesized nanoparticles before and after exfoliation. Photographs of the exfoliated materials are
shown on insets. 1.Wilcoxon,J.P.;Samara,G.A.Phys.Rev.B1995,51,7299-7302 2.Thurston,T.R.;Wilcoxon,J.P.J.Phys.Chem.B1999,103,11-17 3.Wilcoxon,J.P.J.Phys.Chem.B2000,104,7334-7343 4.Ho,W.;Yu,J.C.;Lin,J.;Yu,J.;Li,P.Langmuir2004,20,5865-5869 5.Duphil,D.;Bastide,S.;Rouchaud,J.C.;Pastol,J.L.;Legendrel,B.;Levy-Clement,C.Nanotechnology2004,15,828-832 6.Bai,L.;Ma,X.;Liu,J.;Sun,X.;Zhao,D.;Evans,D.J.Am.Chem.Soc.2010,132,2333-2337 7.Wilcoxon,J.P.;Martin,J.E.;Provencio,P.Langmuir2000,16,9912-9920 8.Krueger,K.M.;Al-Somali,A.M.;Falkner,J.C.;Colvin,V.L.Anal.Chem.2005,77,3511-3515 9.Joensen;Frindt;Morrison.MaterRes.Bull.21(1986)457 10.Heising;Kanatzidis.J.Am.Chem.Soc.121(1999)11720-11732
Copyright © American Chemical Society