AmericanChemicalSociety.com
Reports: B7 45822-B7: Ordered Arrays of Polyphenols by Tandem Reactions
I. David Reingold, Juniata College
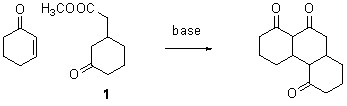
During the summer of 2010 Sarah Border and Robert Parker worked on the Kekulene project. Sarah tried many different approaches to the condensation reaction shown; she was able to reproduce the previous workers' best yield of 10% but was unable to improve on it.
One of the problems we had was that our stock of the
starting ketoester 1 had been
depleted, and attempts to make more were running into problems. Previous students had run this reaction in a
polar aprotic solvent, and workups that removed solvent also removed
product. Distillation also was less than
satisfactory. At the end of the summer,
we finally developed a solventless approach that made the desired compound
quite cleanly with little workup, just a distillation straight out of the
reaction mixture. We now have a large
supply of clean starting material.
There are two problems we have identified with the above cyclization
reaction. The first is that the methyl
ester generates methoxide in the reaction mixture, which can act as a
nucleophile to trigger side reactions such as the Baylis-Hilman reaction. We thought we could prevent this by using the
tert-butyl ester as the starting
material. During the spring of 2010, Katerina
Korch was able to make this ester in good yield by doing a reagentless Krapcho
decarboxylation (polar aprotic solvent but no water or salt added). Unfortunately, the desired reaction did not
occur at all with the tert-butyl
ester.
A second problem is that when treating the above mixture with
base, there are many possible locations for deprotonation to occur, only one of
which leads to the desired product. We
speculated that by making the enamine of the ketoester 1, we could possibly isolate the enamine corresponding to the
correct site for reaction, or, at worst, limit the reaction to two sites. In fact, during the summer of 2010, Robert
Parker was able to make the desired enamine, but could not separate the two
isomers from each other. He was also
unable to make the enamine react with cyclohexenone. We are now trying to make the enol silyl
ether of 1, again assuming that on
treatment with fluoride, it will make the desired enolate and its isomer but
not other enolates.
Copyright © American Chemical Society

