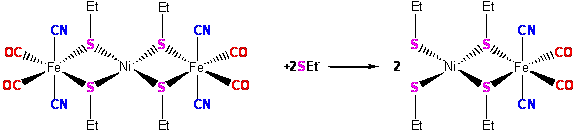AmericanChemicalSociety.com
Reports: UNI3 49424-UNI3: Rational Design and Synthesis of Structural Analog Complexes of the Active Site of Ni-Fe Hydrogenases
Jianfeng Jiang, PhD, Yeshiva University
Introduction
We proposed to design and synthesize of analog complexes the active sites of Ni-Fe hydrogenases.
The synthesis start with fac-[Fe(CO)3X3]- (X = Br and I). We first managed to replace two halides by cyanide and obtained key intermediates fac-[Fe(CN)2(CO)3X]- (X = Br and I). Thiolate bridging Ni-Fe analog complexes [(dppe)Ni(m-SEt)2Fe(CN)2(CO)2]6 (dppe = 1,2-Bis(dipheylphosphino)ethane and SEt = ethanethiolate) and (dppe)Ni(m-pdt)Fe(CN)2(CO)2 (pdt = 1,3-propanedithiolate) were synthesized and structurally characterized. These results have been published. We are currently working on the synthesis of more Ni-Fe analog complexes with the general formula (L2)Ni(m-SR)2Fe(CN)2(CO)2 (L2 = bi-dentate supporting ligand) to study the effects of bridging thiolates and supporting ligands on the structural, stability and electronic properties of the analog complexes. Additionally, we are studying the reactions of intermediates fac-[Fe(CN)2(CO)3X]- (X = Br and I) with thiolates and other ligands.
Results
1. Synthesis of fac-[Fe(CO)3X3]1- (X = Br, I)
fac-[Fe(CO)3X3]1- (X = Br, I) were synthesized in high yield (Br (87%) and I (77%)). Both complexes were structurally characterized to confirm their geometry. These results have been published in Inorganic Chemistry Communications.
2. Synthesis of the Key Intermediate: fac-[Fe(CN)2(CO)3X]1- (X = Br, I)
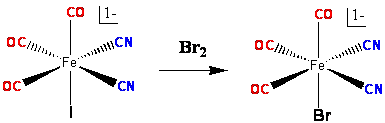
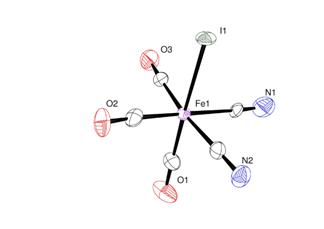
fac-[Fe(CN)2(CO)3I]1- was synthesized by substitution reaction between 2 equivalents of cyanide and fac-[Fe(CO)3I3]1- (Scheme 1) (70% yield). However, fac-[Fe(CN)2(CO)3Br]1- was synthesized by the reaction of fac-[Fe(CN)2(CO)3I]1- with Br2 (Scheme 2) (90% yield). X-ray diffraction experiment has confirmed their structures (Figure 1). Part of these results has been published in Inorganic Chemistry.
Scheme 1: Synthesis of fac-[Fe(CN)2(CO)3I]1- Figure 1: ORTEP drawing of fac-[Fe(CN)2(CO)3I]1- at 50%
probability level
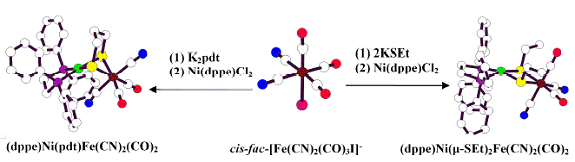
3. Synthesis
of Thiolate Bridging Ni-Fe analog complexes Ni-Fe
analog complexes [(dppe)Ni(m-SEt)2Fe(CN)2(CO)2]6,
(dppe)Ni(m-pdt)Fe(CN)2(CO)2
and (dppp)Ni(m-pdt)Fe(CN)2(CO)2
(dppp = 1,3-Bis(diphenylphosphino)propane
were synthesized and structurally characterized. Synthetic pathway is shown in Figure 2. Part of these results has been published in Inorganic Chemistry. Figure 2: Synthesis of dithiolate bridging Ni-Fe complexes In
(dppe)Ni(m-pdt)Fe(CN)2(CO)2
and (dppp)Ni(m-pdt)Fe(CN)2(CO)2,
the NiS2Fe rhomb are folded with dihedral angle close to 110o, in [(dppe)Ni(m-SEt)2Fe(CN)2(CO)2]6,
the NiS2Fe rhomb are flat with dihedral angle close to 170o. As a result, the Ni-Fe distances are vastly
different (2.8Å for folded structures vs. 3.4Å for flat structure). These results demonstrated that some changes
in bridging thiolates have substantial effects on the
structures of metal centers. On the
contrary, the change of diphosphine supporting ligands (dppe or dppp) does not change the structures of the metal
centers. We will study these effects in
detail with different thiolates and supporting ligands. By choosing
a series thiolates with different steric
and electronic proprieties, we are hoping to figure out what are the factors to
control the geometry of the resulting Ni-Fe dimmer. We will also study the system with supporting
ligands which are less structural hindered to find
out the effects from supporting ligands on the nickel
side. One
of the unpredicting effects is the rearrangement of
CN/CO ligands on iron during the preparation of Ni-Fe
dimers. The
structures of (dppe)Ni(m-pdt)Fe(CN)2(CO)2
and (dppp)Ni(m-pdt)Fe(CN)2(CO)2
showed that the 2 CN- ligands are trans- to one another. Since we started with the cis-CN intermediate fac-[Fe(CN)2(CO)3I]1-, a ligand rearrangement has occurred during its reaction with pdt, Ni(dppe)Cl2 or
during crystallization. It is possible
that an isomeric structure could also be present or could be generated under
different reaction conditions (e.g. doing the reaction and crystallization in
the dark). To illustrate the intrinsic
reactivity of fac-[Fe(CN)2(CO)3X]1-,
(X = Br, I) we are exploring the reaction of fac-[Fe(CN)2(CO)3X]1-, (X =Br, I)
with many ligands such as thiolates,
alkoxides, carboxylates,
amines and phosphines. The reaction of fac-[Fe(CN)2(CO)3I]1-
with triphenylphosphine (PPh3) was studied
in detail.
4. Kinetics study of the reaction of fac-[Fe(CN)2(CO)3I]1-
with PPh3 The
reaction of fac-[Fe(CN)2(CO)3I]1-
with PPh3 showed that one the CO trans-
to iodide can be replaced. Kinetics
study showed that the substitution reaction is first order with respect to fac-[Fe(CN)2(CO)3I]1-
and PPh3. Transition state
enthalpy and entropy changes are obtained. The transition state entropy for
this reaction is positive suggesting a dissociative
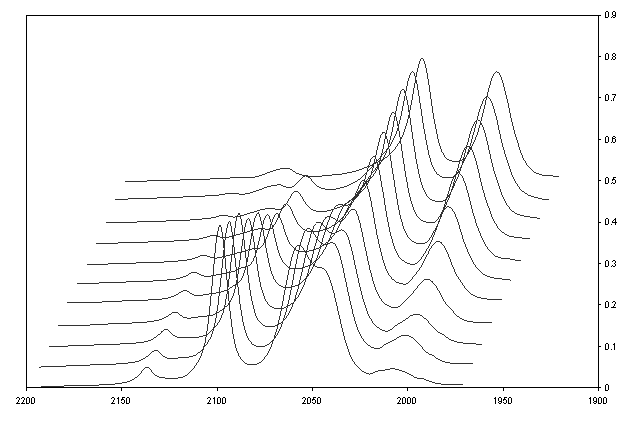
reaction pathway. IR spectra of the
substitution reaction are shown in Figure 3.
A manuscript describing these results was submitted to Inorganica Chimica Acta.
Figure 3: IR spectra of the reaction of 0.020 M fac-[Fe(CN)2(CO)3I]-
with 0.60 M PPh3 in THF at 35.0oC. Spectra were collected every 2 minutes except
for the last one, which was collected 2 hours after the mixing of
reactants. 5. Alternative route to Ni-Fe analog complexes Alternatively, we tried the reaction between fac-[Fe(CN)2(CO)3I]-,
thiolates and Ni2+ in methanol solution in
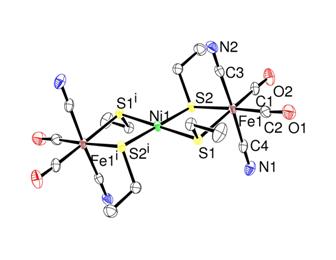
an attempt to make [(RS)2Ni(m-SR)2Fe(CN)2(CO)2]2-. This approach is still underway. However, we have isolated an Fe-Ni-Fe trinuclear complex [Ni[(m-SEt)2Fe(CN)2(CO)2]2]2-
(Figure 4). In this complex, Nickel atom
is coordinated to 4 thiolates. Addition of 2 equivalents of ethanethiolates may producing the desired [(EtS)2Ni(m-SEt)2Fe(CN)2(CO)2]2-
(Scheme 3). This reaction is actively
under investigation. Figure 4: ORTEP
drawing of [Ni[(m-SEt)2Fe(CN)2(CO)2]2]2-
at 50% probability level Scheme 3: Proposed reaction to make [(EtS)2Ni(m-SEt)2Fe(CN)2(CO)2]2- Summary Key
intermediates fac-[Fe(CN)2(CO)3X]-,
(X = Br, I) were synthesized. Thiolates-bridging
Ni-Fe complexes as analog of the active sites of Ni-Fe hydrogenases
were prepared. Effects on the metal
center structures by bridging thiolates and terminal
supporting ligands are studied. The proposed reactions were carried out
smoothly. We are confident that we can achieve our goal in the near future.
With
PRF support, I was able to purchase a much need glove box, which greatly
increased our productivity. I was also
benefited from PRF support during summer time so that I can put 100% effort in
research. With PRF support, I was able
to attend the 239th ACS meeting at 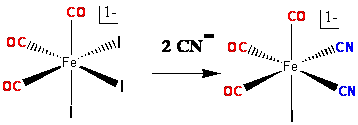
Scheme 2: Synthesis of fac-[Fe(CN)2(CO)3Br]1-
Copyright © American Chemical Society