AmericanChemicalSociety.com
Reports: UNI3 49510-UNI3: Chirality Recognition by Macrocyclic and Multi-Functional Metal Complexes
J. Frantz Folmer-Andersen, PhD, State University of New York, College at New Palz
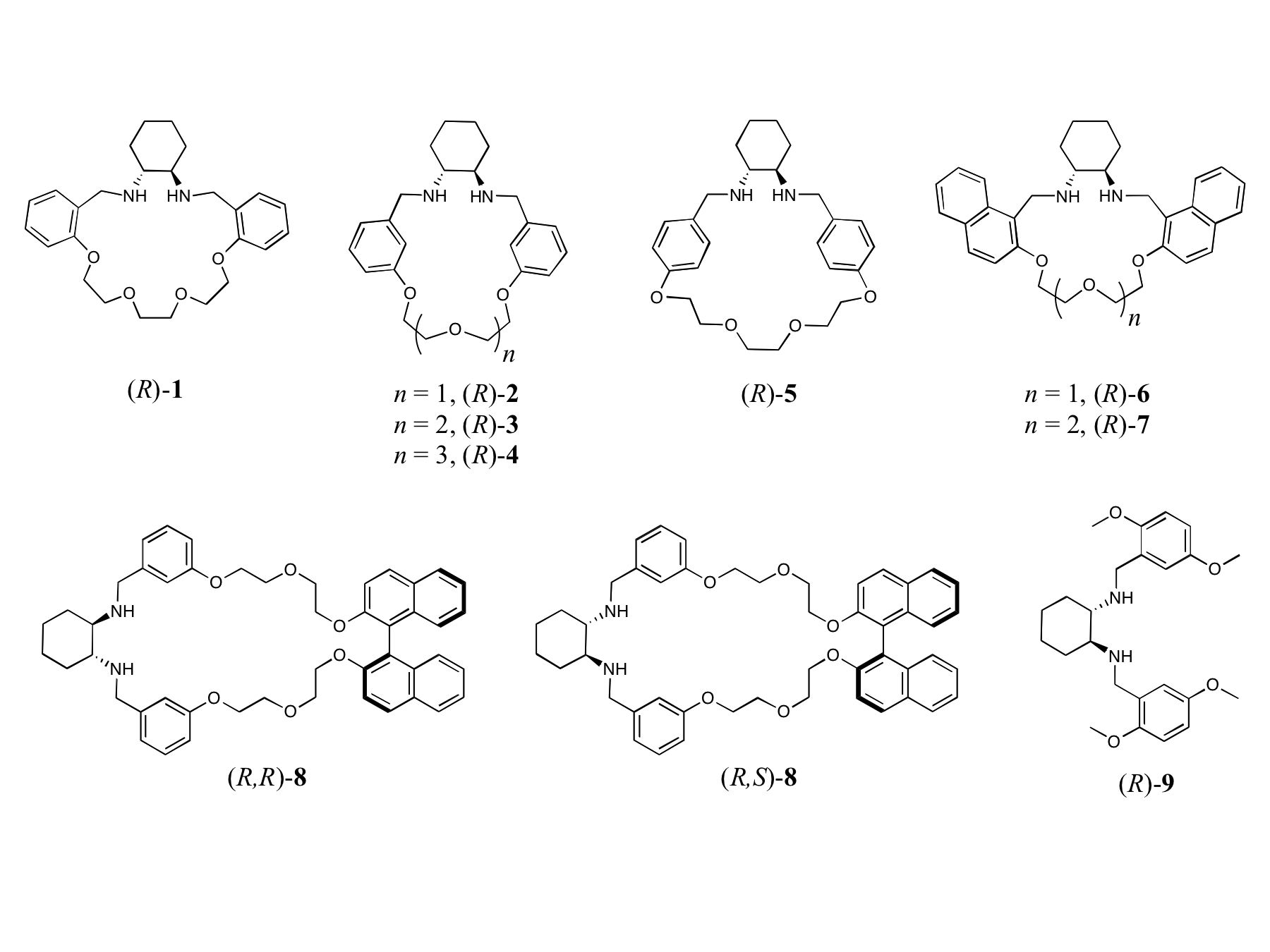
Synthesis. This grant supports the synthesis of chiral, Lewis-basic macrocycles, and the evaluation of their use as enantioselective host molecules, both in free-base form and as labile metal complexes. Over the past year, we have synthesized the trans-1,2-diaminocyclohexane based macrocycles (R)-1 through (R)-7, and have made significant progress towards the isolation of diastereomeric macrocycles (R,R)-8 and (R,S)-8 (see Figure 1). Each of the isolated compounds has been prepared in large quantities (~ 500 mg – several grams), and has been characterized by 1H, 13C and COSY NMR spectroscopy.
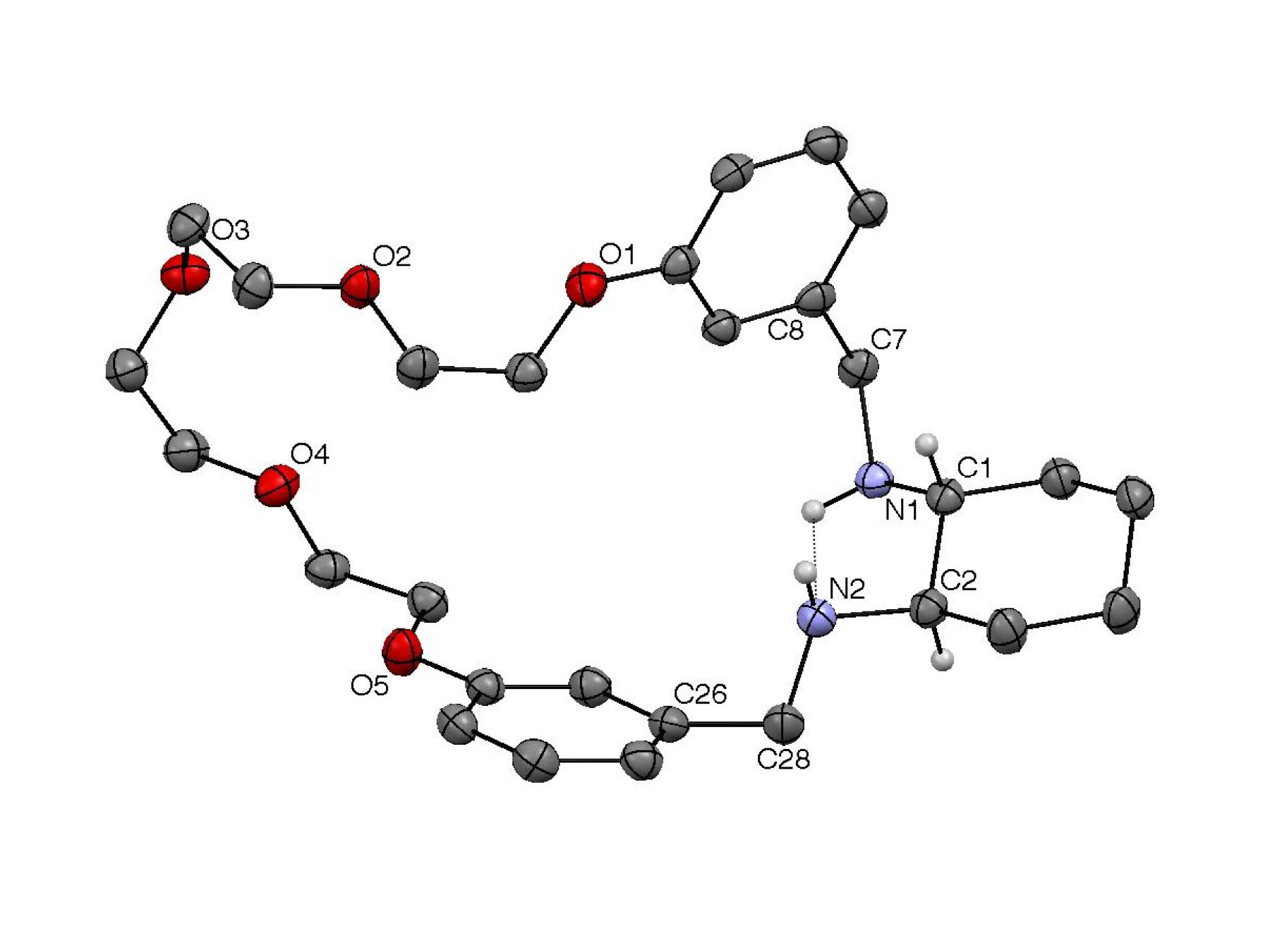
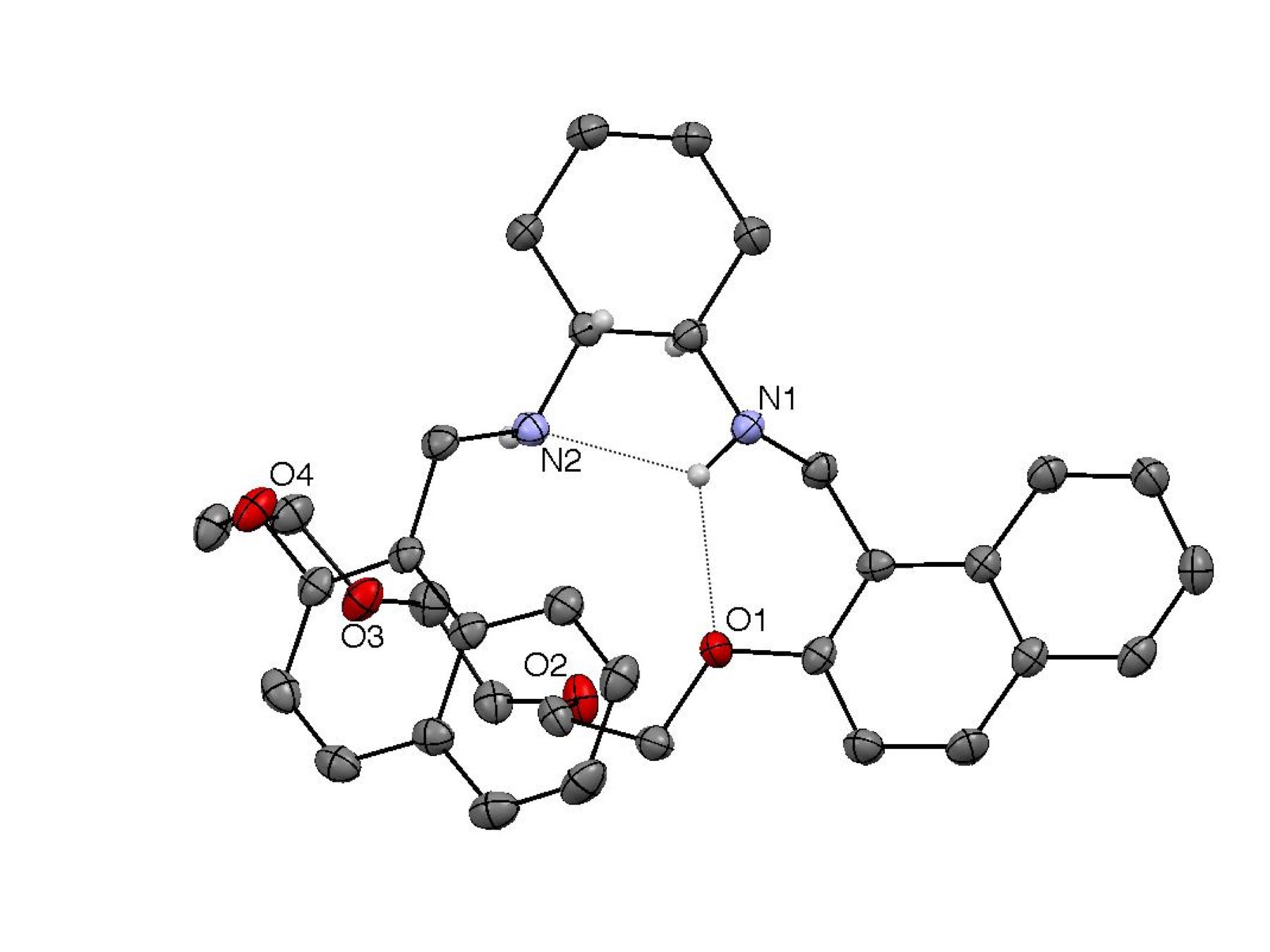
Figure 1. X-ray crystallography and molecular modeling. Compounds (R)-4 and (R)-7 were characterized by X-ray crystallography
(Figures 2 and 3, respectively). Both structures show the same (R,S) configuration of the two stereogenic N atoms to
facilitate an intramolecular hydrogen bond. The arene rings of both structures
are also directed away from the cyclohexyl groups, fashioning a relatively
rigid cleft in which the vicinal diamino moieties reside. The meta linkages of (R)-4 appear to enforce a more extended macrocyclic
structure, whereas the 20-membered ring of (R)-7, which contains ortho linked spacers, exhibits a pronounced kink.
Molecular modeling studies (Monte-Carlo
searching using MM94 force field followed by single point calculations of the
100 lowest E structures at the HF/3-21G level) confirm that the macrocycles
become less planar as the arene linkage geometry is changed from para to meta
to ortho. The optimized structure
of (R)-4 is
virtually superimposable on the crystal structure shown in Figure 2, whereas
the crystal structure (R)-7
is very similar to one of the lowest energy computed structures.
Figure 2. Figure 3. Enantioselective binding of mandelic acid. Several of the macrocycles prepared were observed
by 1H NMR to bind mandelic acid (MA) enantioselectively in
chloroform. The addition of a macrocycle to a racemic solution of MA causes
upfield shifting and splitting of the benzylic C-H signal of MA into two equal
intensity singlets that is greatest when the molar ratio of macrocycle to MA is
near 0.3. Macrocycles (R)-3,
(R)-4, and (R)-7 provide the greatest levels of
enantiodiscrimination and are significantly more effective than the acyclic
receptor (R)-9. Of the
three best macrocycles, (R)-4 is the most effective, with maximal peak separations > 0.1 ppm
achievable. On the other hand, receptors (R)-1, (R)-2, (R)-5, and (R)-6 were less effective than the acyclic control (R)-9in discriminating MA enantiomers. Various
MA derivatives bearing halogen and methoxy substituents on the phenyl ring were
also tested. In general the results confirmed that (R)-3, (R)-4, and (R)-7
are most effective and are capable of providing baseline resolved separation
between enantiomers. No binding by any of the macrocycles to the ester methyl
mandalte is observed, which implies that the carboxylic acid group is involved
in the primary associative interaction. The utility of the present macrocyclic
receptors as chiral NMR shift reagents was demonstrated in the case of (R)-3, which was used to determine the
enantiomeric excess of five MA samples with less than 2% error by integration
of the resolved signals. In all cases, the magnitude of the enantiodiscrimination
was found to increase with total concentration of MA and macrocycle. The poor
solubility of MA in chloroform is not a limiting factor however, because in the
presence of a macrocycle, the solubility of MA is dramatically enhanced. The
specific binding processes were investigated by 1H NMR titrations in
which single MA enantiomers were used as guests. Both continuous variation and
mole ratio studies support the existence of macrocycle:MA complexes of 1:1 and
2:1 stoichiometry, likely resulting from proton transfer from the carboxylic
acid group of MA to the amine group of the receptor, with subsequent ion
pairing. Comparison of analyses of MA enantiomers suggests that for (R)-3 and (R)-4, it is the 2:1 and not the 1:1
complexes, which provide enantioselectivity, whereas for (R)-7, both 1:1 and 2:1 complexes exhibit
enantioselectivity. Future
studies will be aimed at quantifying the 1:1 and 2:1 apparent association
constants, and characterizing the Zn(II) and Cu(II) complexes of the
macrocycles. Preliminary work along these lines suggest that the metal linked
macrocycles (R)-3 and (R)-4 from well defined Zn(II) complexes,
whereas the Collaboration: The
X-ray crystal structures were determined in collaboration with Professor Joseph
Tanski of Vassar College. Much of the macrocycle synthesis was performed by
Philip Atwood (Chemistry, '12), Tyler Moore (Chemistry, '10), and Shaun Ben-Ari
(Chemistry, '12). The majority of the NMR binding analyses were performed by
Thomas Quinn (Biology, '11). Philip Atwwod and Tyler Moore were directly
supported by the present award.
Copyright © American Chemical Society