AmericanChemicalSociety.com
Reports: DNI10 48765-DNI10: Solid-State Dye-Sensitized Solar Cells: Fabrication and Fundamental Investigations
Ashutosh Tiwari, PhD, University of Utah
The objective of this project is to design and characterize a new class of Dye-Sensitized Solar Cells (SS-DSSC) that utilizes solid hole-collectors in place of liquid electrolytes. These solid-state Dye-Sensitized Solar Cells (SS-DSSCs) are expected to be very stable, efficient and scalable for large area applications. The fund is supporting 100% of a graduate student who is getting excellent training in renewable energy materials and photovoltaic research. Project is also supporting 4% of PI's salary (0.5 month summer).
In this project we are utilizing CuBO2, a newly reported p-type oxide, with n-type TiO2 and Ru-based dyes to fabricate SS-DSSCs. Main emphasis is on increasing the conversion efficiency of these SS-DSSCs by optimizing their nanoarchitecture, sensitizing dye and charge injection and recombination dynamics. New material configurations and processing methods are being developed to achieve a better connection between dye molecules and hole-collectors via better pore-filling and contact with the entire TiO2 film. This in conjunction with the high electrical conductivity and hole-mobility of CuBO2 is expected to result in significant improvements in the performance of the solar cell. Further efforts are being made to enhance the conversion efficiency by using coupled dye mixtures.
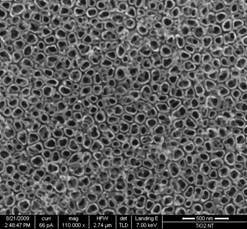
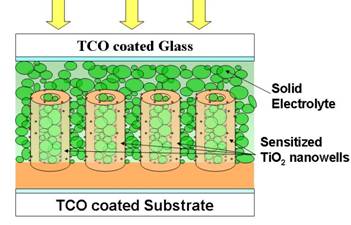
In the first half of the project, we have successfully developed a novel technique of synthesizing nano-well like electrodes for SS-DSSCs. In fig.1 (a) we have shown a SEM image of nano-well like structures prepared by our technique. Walls of these wells are 5-10 nm thick while their bore diameters are in the range 100-150 nm. Because of the unique nanoarchitectures, these electrodes possess very large surface area while maintaining sufficient spacing/opening for loading solid electrolyte in the structure. Using these TiO2 nano-well electrodes we are trying to fabricate SS-DSSCs as shown schematically in fig.1(b).
Fig.1: (a) SEM
images of TiO2 nano-wells, (b) Schematic of our nano-well based
SS-DSSC. Fig.2: Set-up for making TiO2 nanowell electrodes.
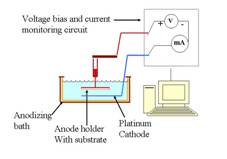
Fig.2 shows a schematic design of our nano-well
fabrication system. The fabrication process includes applying a constant DC
voltage bias to anode that is cleaned, degreased Titanium coated glass held
against Platinum foil as a cathode.
Current that flows through the system is measured by an ammeter as shown
in the schematic diagram of the electrolytic bath. Monitoring and controlling
the current through the system enables us to decide the completion of the
process of formation of nanowells. A constant bias voltage in the range of
10-25V is applied across the electrodes in a 0.5 wt % aqueous solution of HF
acid.
The whole process of formation of nanowells involves
multiple simultaneous reactions broadly classified under two processes: 1)
Oxidation of Ti metal 2) Dissolution of Ti salt
2H2O
à O2 + 4e + 4H+ Ti +
O2 à TiO2 ------(1) TiO2
+ 6F- + 4H+ àTiF2-6 +2H2O ------(2) Initial stage is oxidation of Ti metal that forms a thin
TiO2 barrier layer. The bias voltage
causes localized breakdown of this weak dielectric oxide layer forming pits
that turn into forming the nanowell center.
The Flouride ions break this barrier layer by selectively dissolving it
and forming soluble Titanium salt. The
dissolution rate at the base of the pits is faster than the edges of well due
to localized higher pH. This structure is called anodized structure. Once
anodization is set in it the formation of new oxide layer and its subsequent
dissolution occurs preferentially in the base of the wells extending their
lengths. Anodizing for longer times
leads to formation of deeper well if bath composition and bias voltage is
selected properly. Other bath parameters like temperature, viscosity,
conductivity and mobility of acid cations also affect the rate of formation and
morphology of the wells. All experimental variables finally control the rate of
Titanium oxide growth or its dissolution and diffusion of the ionic species.
Fig.3: SEM images of a TiO2 nano-well filled with NiO nanoparticles.
We are also working on optimizing a method to fill TiO2
nanowells with solid electrolyte nanoparticles. In this method, TiO2
nano-well electrodes are impregnated with the nanopowders of solid electrolyte
by the capillary effect. For this, a suspension of dispersed electrolyte
nanoparticles in pure ethanol is added drop wise to the sensitized electrodes,
which are ultrasonicated to disperse the suspension evenly over the
electrode. This process is repeated
several times to fill the nano-wells with solid electrolyte particles (see
fig.3).
Experimental preparations are also going on to investigate the
electron injection and recombination dynamics in CuBO2-based DSSCs using transient absorption and emission studies and
correlate those with device photovoltaic performance.
Copyright © American Chemical Society