AmericanChemicalSociety.com
Reports: AC5 47369-AC5: Thermally Stable, Sintering Resistant Catalysts
Micha Asscher, The Hebrew University of Jerusalem
As stated in our previous report, we have developed a unique way of growing and controlling bimetallic Pd-Au nano clusters that were subsequently used as our model system to examine chemical reactivity and stability via conversion of acetylene to either ethylene or benzene. We have made significant progress towards studying the effect of surface structural defects on the thermal stability and overall performance of our working catalyst.
This research has significantly improved our understanding of basic issues concerning supported metal clusters interaction with their oxide support.
While the main body of this research has been performed by a graduate student, Mr. Elad Gross, in recent months a new student has started his graduate research in order to proceed and advance this topic. We all attempt to enhance our basic understanding of such fundamental processes in heterogeneous catalysis.
Elad Gross is now well prepared and will join a leading laboratory in UC Berkeley as a Post-Doc by the end of 2010, where he plans to integrate heterogeneous with homogeneous catalysis, attempting to search for fossil fuel alternatives.
1. Auger spectroscopy characterization of bimetallic clusters on sputtered, defected SiOx surfaces
The emphasis of our study during this year has been to characterize silicon oxide surfaces after being sputtered by energetic argon ions in order to prepare different types of surface defects prior to bimetallic clusters preparation. Auger electron spectroscopy (AES) has been used to detect the ratio of metallic elements at the top surface of the clusters, following surface annealing, as shown in Figure 1.
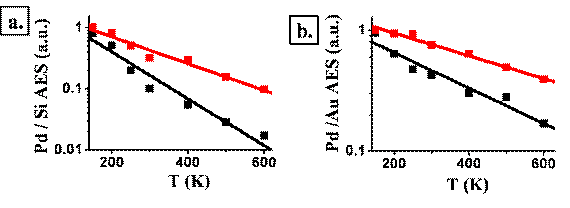
Figure 1: Pd-Au alloy clusters were prepared on native, smooth (black line) and sputtered (red line) SiO2 substrate by simultaneous evaporation of 2Å Pd and 2Å Au on 30ML H2O and annealed to different temperatures. Pd/Si (a) and Pd/Au (b) AES peak intensity ratios were plotted vs. annealing temperature after clusters deposition.
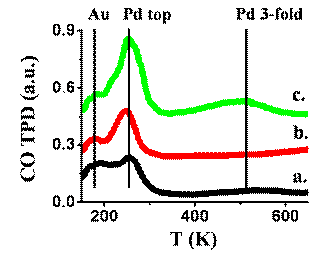
Figure 1a reveals that Pd atoms stay well spread on the surface at much higher temperatures as a result of preparing the silicon oxide surface to become "defects rich" using the argon ion sputter. As better resolved by employing CO-TPD measurements, the bimetallic clusters are simultaneously more thermally stable on the defected surface and modified in their surface structure, including higher surface density of Pd. The CO-TPD spectra shown in Figure 2 demonstrate how the CO desorption peak near 250K, associated with Pd atoms at the top surface, significantly increases in intensity relative to the peak associated with Au atoms, after sputtering the SiO2 surface prior to bimetallic clusters deposition.
Figure 2: CO TPD measurements following exposure of 30L CO over Pd-Au
clusters prepared on native oxide (a.) and sputtered SiO2 substrate at beam sputter
energies of 1.5 (b.) and 2.5KeV (c.).
2. Reactivity studies on top of defect-rich silicon oxide support
The fact that defects can lead to structural modification of the bimetallic Pd-Au clusters such that Pd atoms segregate to the surface is a new and most important observation. The outcome of such an effect is that the reactivity of clusters that have been deposited on top of the defected is expected to modify not only in terms of overall reactivity, but also with respect to selectivity.
This issue has been addressed by comparing the reactivity of our model reaction: acetylene hydrogenation to ethylene and trimerization to benzene over the smooth and defect-rich silicon oxide samples. In Figure 3 we summarize the results of reactivity studies over the different types of support surfaces.
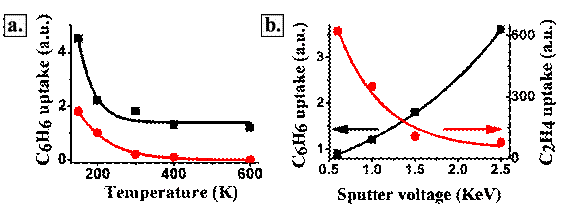
Figure 3: a. Benzene formation at the indicated substrate annealing temperatures. Benzene uptake was measured following adsorption of 3L C2H2 at 120K at different pre-annealing temperature, the clusters were prepared on smooth native SiO2/Si(100) (red) and on sputtered SiO2/Si(100) substrates (black). b. Benzene (black) and ethylene (red) formation as function of the sputter beam voltages
We have observed that indeed the overall reactivity towards ethylene conversion reactions could be maintained to higher temperature (up to 600K, Fig. 3a for benzene formation). But at the same time the selectivity towards ethylene formation that was at a ratio of 1:100, significantly changes in favor of benzene over the defect-rich substrate (Fig. 3b).
We attribute this significant change in the pattern of reactivity to the modified surface segregation of Pd atoms. This process makes the Pd atoms more abandon on the bimetallic cluster surface, resulting in enhanced reactivity towards benzene formation. We understand this morphology change in the formation of larger patches of extended Pd (111)-like (hexagonal) surfaces that are known to preferentially enhance benzene formation from acetylene.
3. Nature of defects on the silicon oxide surface
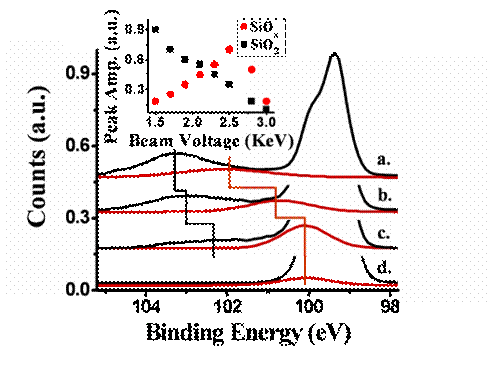
Finally, a further investigation for the possible origin of defects, formed by the energetic argon ion sputter and their characterization, has been carried out by performing in-situ XPS analysis of gradually sputtered silicon oxide surfaces at different argon ion kinetic energies. This is summarized in Figure 4.
Figure 4: XPS spectra of Si 2p, SiO2 (black) and deduced SiOx (red) signal following substrates Ar+ ion sputtering at beam voltages of 1.75 (a.), 2.0 (b.), 2.5 (c.) and 2.75KeV (d.). Inset: SiO2 and SiOx peaks amplitude as function of the sputter voltage energies.
It is clearly shown in Figure 4 that as one increases the argon ion kinetic energy during surface collision (sputter), a characteristic new XPS peak gradually emerges near 103 eV. This new peak draws intensity from the main SiO2 peak (silicon signal near 99.5 eV). At this point it can tentatively be assigned to a mixture of SiOx sites that represent an electronically reduced silicon.
These results were summarized and will be submitted for publication within 2-3 weeks.
Copyright © American Chemical Society