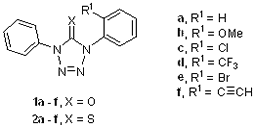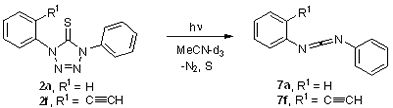AmericanChemicalSociety.com
Reports: G4 48202-G4: Substituent Effects on the Photochemistry of 1,4-Disubstituted Tetrazolethiones
Sundeep Rayat, Kansas State University
A.1 Syntheses of tetrazolethiones 1a – f and 2a – f
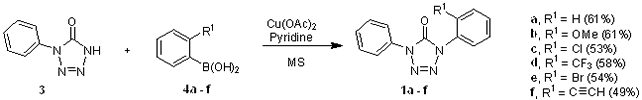
We synthesized 1,4-diaryl tetrazol-5-ones 1a – f by the copper mediated coupling of a series of ortho substituted boronic acids 4a – f (R1 = H, OMe, Cl, CF3, Br, CºCH) with 1-phenyl-1H-tetrazol-5(4H)-one (3) in the presence of pyridine and molecular sieves (Scheme 2). The boronic acids 4a–e were commercially available while 4f was prepared from phenyl acetylene by a reported method and used without further purification due to its low yields.
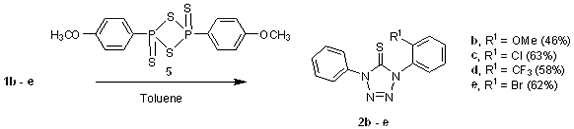
Treatment of 1b – e with Lawesson’s reagent (5) yielded the corresponding 1,4-diaryl tetrazole-5-thiones 2b – e (Scheme 3).
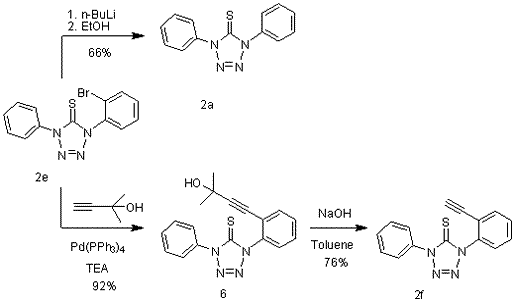
A.2 Photochemistry of tetrazolethiones 2a and 2f
Photolyses of 2a and 2f were carried out in a Rayonett reactor at 254 and 300 nm in acetonitrile-d4. The reaction mixture was analyzed by NMR spectroscopy. The primary photoproduct was the carbodiimides 7a and 7f, respectively formed by the loss of dinitrogen and sulfur (Scheme 5).
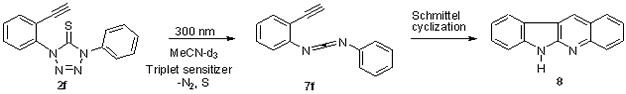
We wished to explore whether the presence of an ethyne moiety ortho to a tetrazolethione ring in 1-(2-ethynylphenyl)-4-phenyl-1H-tetrazole-5(4H)-thiones (2f) could allow the photochemically generated carbodiimides to further engage in a Schmittel reaction under triplet sensitization to form the indoloquinolines 8. To our delight, 2f underwent clean conversion to the expected indoloquinolines 8 upon irradiation in the presence of triplet sensitizers (Scheme 6).
A.3 Summary
Overall, these studies indicate that tetrazolethiones are highly promising lead compounds for industrial, agricultural and medicinal applications. The search for derivatives with improved quantum yields that retain the clean photochemistry and end product photostability of tetrazolethiones would be of considerable interest. Our mechanistic studies suggest that derivatives should be sought that favor dinitrogen dissociation in the excited state, since this appears to be the favored pathway in the photodecomposition of these compounds.
A.4 Impact of this award
The major impact of this award has been in providing insights into the mechanism of photodecomposition of tetrazolethiones and the highly clean photodecomposition of these compounds to a photostable end product makes them promising lead structures for industrial, agricultural and medicinal applications. The results generated as a result of this award were presented in contributed lectures/posters by the PI’s students/postdoc at the 44th Midwest Regional Meeting of the American Chemical Society held in Iowa City, IA, from October 21-24, 2009, NOBCChE 37th Annual Conference held at Atlanta, GA from March 29th-April 2nd, 2010 and at the Reaction Mechanism Conference held at the University of Massachusetts, Amherst from June 23-26, 2010. PI was also invited to give a talk at the 20th Winter Inter-American Photochemical Society Meeting held at St. Pete Beach, Florida from January 2nd-5th, 2010. We have already published one manuscript on the results obtained as a result of this award and a second one will be submitted in next few days. The presentations at the national/international meetings and publications in peer-reviewed journals has allowed my research group visibility in the scientific community and enabled us to obtain positive feedback and constructive criticisms to further refine our research ideas. All of this would have otherwise been not possible without the support from ACS-PRF.
Copyright © American Chemical Society