AmericanChemicalSociety.com
Reports: UNI7 49338-UNI7: A Systematic Approach Towards the Synthesis of Metal Coordination Cross-Linked Polymer Networks
Alexander D. Schwab, Appalachian State University
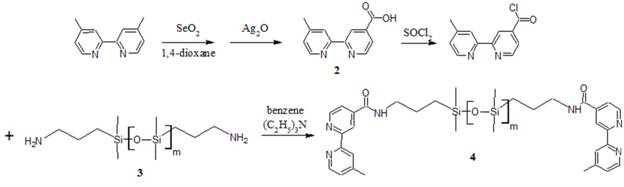
The overall goal of this project is to synthesize an elastomeric (rubber) material where intermolecular cross-links are composed of metal-coordination complexes rather than covalent bonds. To achieve this goal, poly(dimethyl siloxane) molecules terminated with metal binding ligands have been synthesized. The first strategy, shown in the following figure, was implemented by an undergraduate chemistry student, Sarah Miller in the summer of 2009.
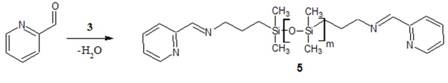
Also in the summer of 2009, another undergraduate chemistry student, Scott Meadows, was able to synthesize a different ligand-terminated PDMS molecule by the method illustrated in the following figure.
The 1H NMR spectra of the polymeric products (4 and 5) indicate the syntheses were successful. Characterization with gel permeation chromatography (GPC), however, has been problematic. Compounds 3, 4, and 5 exhibit different retention times on our GPC system when eluting with chloroform though they are expected to have similar molecular weights. Suspecting column/polymer ineractions, triethylamine (TEA) was added to the eluting solvent in increasing amounts causing the retention times move towards agreement. Unfortunately, the TEA eventually masks our detection scheme (UV absorbance and evaporative light scattering). Mr. Meadows and Ms. Miller traveled to Oak Ridge National Lab (ORNL) to use the GPC systems in the Center for Nanophase Materials Science (CNMS) to learn that the ligand attachment to PDMS causes polymer and solvent refractive indices to nearly match, defeating their instruments' detection scheme (light scattering and refractive index).
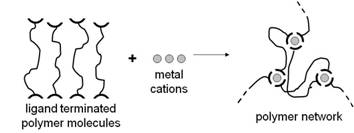
In the next phase of the synthesis, the ligand terminated polymer molecules are cross-linked through the addition of metal cations. If more than two ligands coordinate to each metal cation, a three-dimensional polymer network as found in conventional elastomeric materials can eventually be realized as illustrated in the following figure.
In the fall of 2009 and the spring of 2010, Mr. Meadows worked on developing a metal coordination cross-linking scheme that would be suitable for cross-linking product 4 using non-polymeric bipyridine ligands. Ms. Miller continued this work in the summer of 2010 and eventually attempted to “cross-link” 4. The procedure yielded an insoluble particulate when carried out in dilute solution.
This fall, Kurtis Price, an undergraduate student in chemistry, is working to streamline our lengthy workup of product 4, and is modifying our GPC system with new low-interaction columns to better characterize polymer size without polymer/column interaction effects.
Once the ligand-terminated polymer molecules can be fully characterized, future work will include the development of a concentrated solution or melt procedure to truly realize a monolithic elastomeric object. Characterization of this “object” may then be limited to UV/vis spectroscopy and viscometry (also available at ORNL CNMS) to determine the effectiveness of the cross-linking reaction.
Copyright © American Chemical Society