AmericanChemicalSociety.com
Reports: AC3 47214-AC3: Oxygen Activation by Isopenicillin N Synthase - The Yin and Yang of C-H Activation
Carsten Krebs, Pennsylvania State University
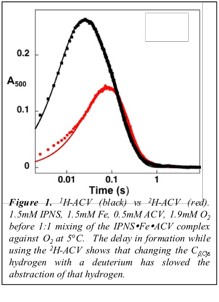
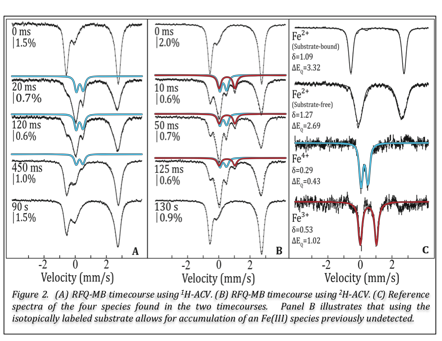
During the previous two funding periods, we successfully trapped and characterized a mid-valent Fe(III) and a high-valent Fe(IV) intermediate along the isopenicillin N synthase reaction pathway with oxygen. The spectroscopic parameters suggest that the first intermediate is the first hydrogen-abstracting intermediate, a Fe(III)-superoxo complex while the second is a Fe(IV)-oxo. The Fe(IV)-oxo intermediate was detectable via stopped-flow absorption and rapid freeze-quench Mšssbauer spectroscopy using the native substrate, d-(L-α-aminoadipoyl)-L-cysteinyl-D-valine. However, it was necessary to use kinetic manipulation via an isotopically labeled substrate at the Cβ,Cys, the first hydrogen abstraction site, A-(b-[2H]2-C)-V, to trap and characterize the Fe(III)-superoxo intermediate. (Figures 1 and 2)
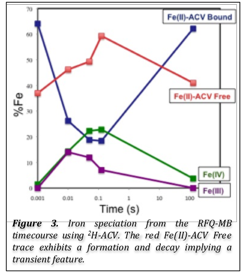
As mentioned in the previous update, a more thorough analysis of the Fe(IV)-oxo SF-abs and MB data uncovered a possible uncoupling reaction. A new species appears to form with similar spectroscopic features as the free-Fe(II) starting material (Figure 3). The working hypothesis is that approximately 50% of the enzyme undergoes an uncoupling step after forming the Fe(III) intermediate. Instead of progressing down a productive pathway to form the β-lactam ring, IPNS forms a new Fe(II) species, possibly an Fe(II)-thiocarboxylate, and forms a product, other than isopenicillin N, before releasing the enzyme for another turnover.
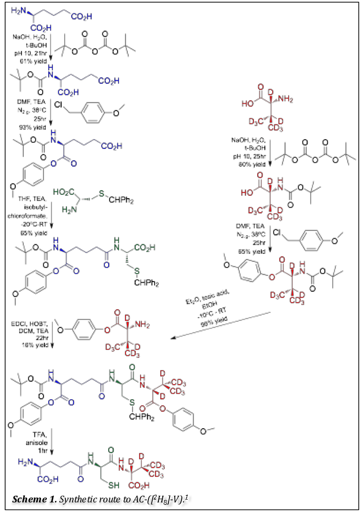
In an effort to investigate this uncoupling pathway, our focus for this funding period has been directed at synthesizing isotopically labeled ACV, deuterated at the second hydrogen abstraction site, AC-([2H]8-V). Because the C-D bond is more difficult to cleave than a C-H bond, the hydrogen abstraction should be slower, resulting in greater accumulation of the Fe(IV) intermediate. With a greater accumulation of the Fe(IV)-oxo, we will be able to more fully characterize the electronic structure of the intermediate, including the spin state of the iron, the zero-field splitting parameters D and E/D, and the 57Fe A-tensor.
However, it is also possible that because C-D bond is more difficult to cleave, the equilibrium between the two pathways will shift in favor of the uncoupled reaction. If the off-pathway is favored, this will allow us to further investigate the uncoupled reaction and determine the IPNS by-product, giving us a better understanding of the IPNS mechanism in general.
At this point, we are at the last stages of synthesizing the labeled substrate, AC-([2H]8-V, according to a published procedure.1 Scheme 1 illustrates the steps involved in the synthesis. Once completed, we will perform stopped-flow absorption spectroscopy and rapid freeze-quench Mšssbauer spectroscopy using the AC-([2H]8-V), as was done with the A-([2H]2-C)-V labeled substrate.
Additionally, once we have confirmed that the AC-([2H]8-V) has been synthesized correctly, we will then have the ability to synthesize any additional substrate that will aid in our IPNS investigation.
Reference:
1. Grummit, A.R.; Rutledge, P.J.; Clifton, I.J,; Baldwin, J.E. "Active-site-mediated elimination of hydrogen fluoride from a fluorinated substrate analogue by isopenicillin N synthase" Journal of Biochemistry 2004, 382, 659-606.
Figures:
Copyright © American Chemical Society