AmericanChemicalSociety.com
Reports: B7 44893-B7: Aggregation and Liquid Crystal Properties of Two Chromonic Systems
Peter J. Collings, Swarthmore College
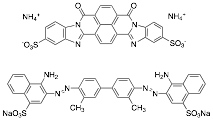
The goal of the grant was to investigate two new dyes that form chromonic liquid crystals, Bordeaux Dye and Benzopurpurin 4B (top and bottom, respectively, in the figure below). The former has a structure quite different from the other dyes previously studied and the latter is reported to have a liquid crystal phase at very low concentrations. These studies were completed a year ago, but a final year was necessary to finish work done on an infrared absorbing dye and begin experiments on the kinetics of the aggregation/disaggregation process.
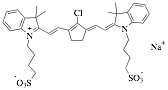
An Infrared Absorbing Dye By this time a year ago, we had mapped out the phase diagram
of IR-806, an infrared absorbing dye that forms a liquid crystal at very low
concentrations. Its structure is
given below.
We had also observed that its absorption spectrum is much
more sensitive to aggregation that the other chromonic
systems. This year we extended the
absorption measurements to higher concentration, only to discover that the last
stage of aggregation, the one that results in the liquid crystal phase changes
the absorption spectrum even more.
Thus this compound allows us to follow the aggregation process with more
specificity that ever before. It
is clear that well below the liquid crystal phase, aggregation occurs, probably
to a dimer or small aggregate. Then at a higher concentration, large
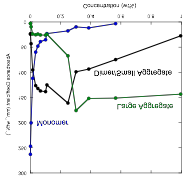
aggregates form resulting in the liquid crystal phase. Three representative spectra are shown
below, illustrating the various changes that take place with concentration. The concentrations are, from left to
right, 0.0005 wt%, 0.101 wt%, and 0.966 wt%.
Also shown in the figure are the results of decomposing the
spectra into individual peaks.
Spectra over the entire concentration range could be fit with 6 peaks (center
wavelength and width fixed), with 3 of these peaks being very sensitive to
aggregation. The concentration
dependence of the amplitudes of the peaks representing the monomer, dimer or small aggregate, and large aggregate are shown in
the figure below.
The fall of the monomer peak, rise and fall of the dimer peak, and rise of the aggregate peak all follow the
prediction of a simple theory in which the equilibrium constant for dimer formation is much larger than the equilibrium
constants for larger aggregates, which are independent of aggregate size. We will submit these results for
publication as soon as the full analysis is completed.
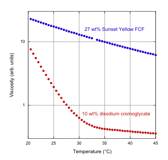
Viscosity Measurements In a collaboration with David Pines
at New York University, we have measured the viscosity of two chromonic liquid crystals as aggregation proceeds and the
liquid crystal phase is formed.
The measurements were taken with a unique apparatus, built completely by
the Pines group, and the experimental precision is outstanding. We chose two systems for which there is
evidence that the aggregation process is very different. As can be seen from the following
figure, the viscosity data also show the great difference between these two
compounds as the temperature approaches the liquid crystal transition (about
20°C in the figure).
There has been little experimental work on the viscosity of chromonic liquid crystals, and we intend to continue this
collaboration.
Kinetic Experiments Investigations into the kinetics of large molecules have
revealed a great deal about their behavior and structure, yet there has been no
experimental work on the kinetics of aggregation and disaggregation in systems
that form chromonic liquid crystals. For this reason, we decided to perform
some preliminary experiments to see if an extensive investigation would be
worthwhile. Slow
kinetics experiments can be done by changing the conditions and monitoring a
sensitive parameter such as absorption or fluorescence. Fast kinetics requires specialized
equipment such as a stop-flow or temperature-jump apparatus. When we realized that the kinetics in
some of the aggregating dyes was too fast for our apparatus, we entered into a collaboration with Dr. Heinrich Roder
at the Fox Chase Cancer Institute, which is located on the other side of
Philadelphia from Swarthmore College.
What we found was a huge variation in kinetics among different
aggregating systems. For Benzopurpurin 4B, which forms a very
large aggregate, reaching a new equilibrium after dilution took hours. For Sunset Yellow FCF, with an
aggregate structure consisting of a stack of molecules, the time to reach
equilibrium after salt is added to promote aggregation was about a
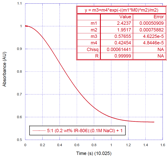
millisecond. For IR-806,
aggregation took about half a second and the absorption vs. time data are fit
so perfectly by a stretched exponential that the fit cannot be distinguished from
data.
A stretched exponential is indicative of a system in which
many time constants contribute to the kinetics. This is very reasonable for the IR-806 system, in which
there are many different sized aggregates, perhaps with varying time
constants. We feel studying the
kinetics of these aggregating dyes is a wide open
avenue of investigation and plan to assemble the resources for an extended
project.
Copyright © American Chemical Society