AmericanChemicalSociety.com
Reports: DNI10 49301-DNI10: Fabrication and Investigation of Porous Tin Oxide Anodes for Li-Ion Micro Batteries
Chunlei Wang, Florida International University
The requirement of higher energy capacity microbatteries demands the exploitation of new substitute materials with higher energy capacity than traditional graphite. SnO2 has been considered as one of the most promising substitutes for the carbon anode in Li-ion batteries due to its high Li+ storage capacity. However, the practical application of SnO2 as anode is restricted by poor cyclability and rate capability due to large volume change during cycling, which can cause disintegration and electrical disconnection from current collector. In this project, we prepared and tested tin oxide anode films with a variety of porous morphologies using Electrostatic Spray Deposition (ESD) technique. Material characterization and electrochemical analysis were conducted in order to investigate the correlation between morphology and electrochemical performance and understand the underlying mechanism. During the past funding year, we have also built two LabView controlled ESD systems with the capability to fabricate porous and fibrous transition metal oxide and composite electrodes with different dimensions. This research has significantly enhanced our understanding of fundamental issues regarding intrinsic properties of porous and fibrous tin based materials as anode for Li-ion batteries. Two postdoctoral researchers, 2 graduate students and 1 undergraduate student were involved in this project.
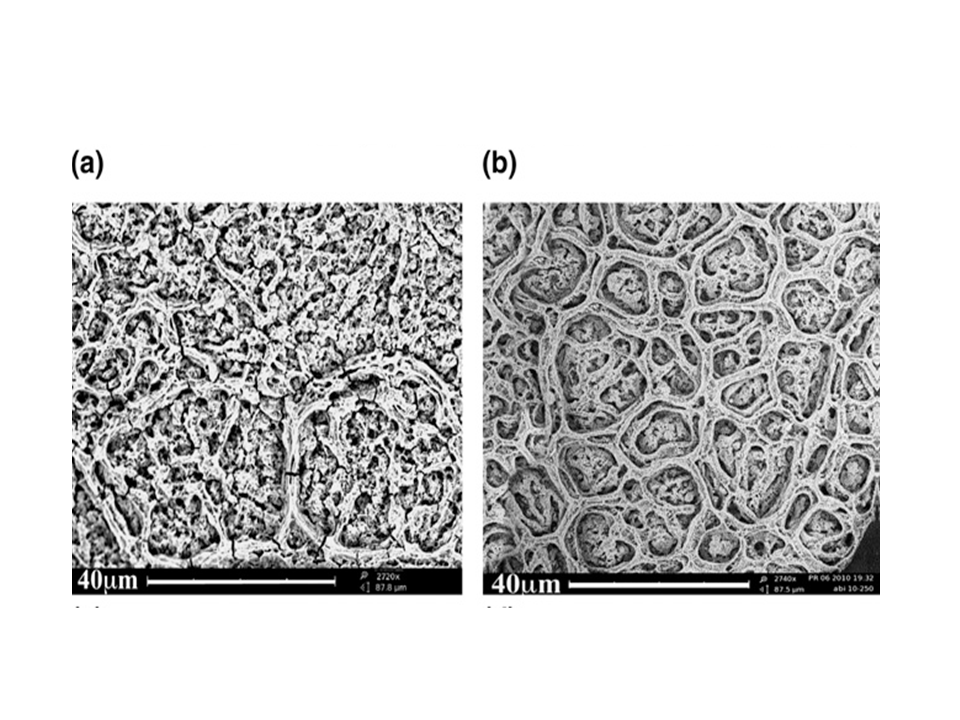
Porous SnO2/multiwalled carbon nanotube (CNT) thin film composites as anode material for Li-ion batteries were prepared using the ESD technique. The morphologies of the samples were found to be affected mainly by deposition temperatures. Electrochemical test cells were assembled using the as-prepared samples without any conductive additive or binder. The influence of deposition temperature and CNT content on the electrochemical performance of the anodes was investigated. Compared to pure tin oxide and pure CNT, the composite anode materials showed better discharge capacity and cyclability. Among the composites, the sample deposited at 250 oC with 30 wt% CNT content was found to show better energy capacity. This can be ascribed to the porous nature of the anodes and the improvement in the conductivity by the addition of CNTs.
Figure 1. Typical SEM images of (a) SnO2/20% CNT sample deposited at 300oC and (b) SnO2/10% CNT sample deposited at 250 oC. A Sn/C composite structure, namely Sn@carbon encapsulated in
bamboo-like hollow carbon nanofibers,
was fabricated by pyrolysis of TBT (core)/PAN (sheath)
nanofibers through a coaxial electrospinning method. As a potential anode
material, this composite displays a high reversible capacity of 737
mAhg-1 after 200 cycles at 0.5 C. It also exhibits a reversible
discharge capacity as high as 480 mAhg-1 when cycled at 5 C . The
particular Sn@carbon encapsulated in hollow carbon nanofibers structure has
high
Sn content (close to 70 wt% Sn and 30 wt% carbon) and provides
appropriate void volume to respond to the large volumes change and to prevent
pulverization of the Sn nanoparticles. This composite is very promising as a
potential anode material for LIBs even though the kinetics of the charge process
requires further improvement. Moreover, coaxial electrospinning has proved
itself to be a powerful routine for the preparation of nanomaterials with hollow
core/shell architectures.
In addition to tin based anodes, we have extended our research
to porous NiO anodes. An interwoven core–shell structured Ni/NiO anode for
lithium ion batteries was created by a simple oxidation of Ni foam. As-prepared
configuration has a high specific discharge capacity of 701 mAh g-1
at the 2nd cycle. Its electrochemical performance at subsequent cycles shows
good energy capacity of 646 mAh g-1 at the 65th cycle as well as good
rate capability, as shown in Figure 2. The porous core–shell structure not only
buffers the volume change during cycling but also effectively increases the
contact among anode, current collector and electrolyte. The small contact
resistance between NiO and Ni facilitates enhanced intrinsic kinetics from
conversion reaction.
Figure 2. (a) Cyclic voltammetry of NiO anode electrode: (i) the 1st cycle, (ii) the 2nd cycle and (iii) the 10th cycle, compared with (iv) the 1st cycle of Ni foam at a scan rate of 0.2 mV s-1. (b) Charge/discharge curves of the 1st, 2nd, 10th, 30th, 50th and 65th cycles at the current density of 156 mA g-1. (c) Cycle performance of NiO anode electrode (3.20 mg) at the current density of 156 mA g-1. (d) Rate capability of NiO anode electrode at different rates: (I) 198, (II) 317, (III) 437, (IV) 556, (V) 675, (VI) 833, (VII) 992, (VIII) 1151, (IX) 1310, and (X) 198 mA g-1.
Copyright © American Chemical Society