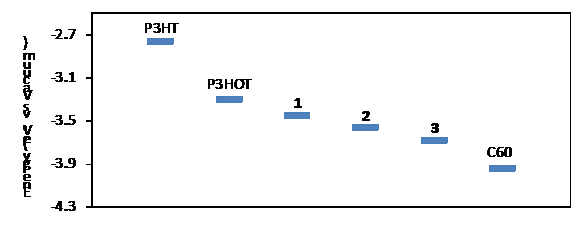AmericanChemicalSociety.com
Reports: ND7 48968-ND7: Design and Synthesis of n-Type Conjugated Polymers with Exceptionally High Electron Affinities for Photovoltaic Application
Li Jia, The University of Akron
Goal
The goal of the PRF-supported research is to design and synthesize p-conjugated polymers with superior n-type semiconducting property (i.e., high electron conductance, s), which has been long sought after but remained sparsely achieved. Two parameters, charge carrier density (n) and mobility (m), must be taken into consideration since s = nm. Under PRF-ND support, we have explored two venues to achieve this goal. First, we used 9,10-diboroanthracene as a key building block that induces high electron affinity. The empty p-orbitals of boron atoms are integrated into the p-system to lower the energy of the lowest unoccupied orbital (LUMO), facilitate electron injection, and increase n. The flat large anthracenoid structure is expected to induce interchain p-interaction and therefore be beneficial for m. In the second direct, we attempted to take advantage of the high ambipolar charge mobility in regioregular polythiophene and incorporated electron-withdrawing groups to the side chain to increase n. The effort has resulted in one published paper and one in press. Our main findings are summarized below.
Results
Diboroanthracene-Containing Polymers. A series of polymers (1, 2, and 3) containing 9,10-Dihydro-9,10-diboroanthracene have been synthesized. Their chemical structures have been characterized using 1H , 13C, and 11B NMR spectroscopy and by comparing with their small molecule analogues which are also synthesized in this work. The molecular weights of 3 have been
characterized
by GPC (Mn = 5050, PDI = 1.14).
It was impossible to carry out GPC for 1
and 2 because of their hydrolytic
instability. Optical
absorptions of polymers 1–3 have been studied. These polymers are
generally wide-gap materials with absorption maximum in the UV and blue region
in non-coordinating solvent or in the solid state. The reduction potentials of 1–3
were measured using cyclic voltammetry to evaluate the electron affinity of the
electron-deficient polymers and the small molecules. The LUMO energies of 1–3
can be estimated from the onset potential of their reduction waves in their
Lewis base-free forms according to a scale factor that relates the SCE
potential to vacuum (4.4 eV is typically used ) and are compared with those of
C60 and regioregular poly(3-hexylthiophene (rr-P3HT) in Figure 1. Apparently,
1–3 are less electron-accepting than C60, but their LUMO
levels are all lower than that of rr-P3HT
by an appreciable margin. Figure
1. Comparison of LUMO energies of polymers 1–3
with C60 and rr-P3HT. Because a major motivation of the present research is to
find a polymeric material to replace the fullerene derivatives in photovoltaic
applications, we have carried out the photoluminescence studies to probe the
possible electron transfer from the excited rr-P3HT
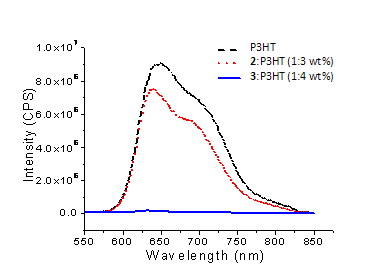
to 2 or 3. The photoluminescence intensity of rr-P3HT in its blend with 2
is only marginally lower than that of pure rr-P3HT
(Figure 2). Insufficient energy offset
at the LUMOs of 2 and rr-P3HT appears an easy culprit to the
lack of quenching. In
Figure 2. Photoluminescence spectra of pure rr-P3HT, a blend of 2 and rr-P3HT, and a
blend of 3 and rr-P3HT. The samples were
excited at 530 nm. comparison,
the photoluminescence of rr-P3HT
in its blend with 3 is quenched to
< 1% of the level of the pure rr-P3HT
sample. This indicates a strong
interaction between the two components in the excited states and rapid electron
transfer from the excited rr-P3HT to 3.
The UV-visible spectra of the blends are simple additions of the
individual spectra of the blend components, and no new bands are observed up to
1100 nm. Hence, the ground states of the
pristine polymers at least appear to remain the most populated states in the
blend. Although the detailed mechanisms involved in the non-radiative decay of
the excitons is unknown at the present stage, to the best of our knowledge, 3 is the first polymer that has been
demonstrated to quench the photoluminescence of rr-P3HT. Polythiophene with Enhanced
Electron Affinity. Head-to-tail regioregular poly(3-heptanoylthiophene) (PHOT)
was synthesized by Ni-catalyzed polycondensation of the

2,2-dimethyl-1,3-propanediol-protected Grignard monomer followed by deprotection. Cyclic voltammetric study demonstrates that the highest occupied orbital (HOMO) and lowest unoccupied orbital (LUMO) of PHOT are 0.5 eV
lower in energy than those of rr-P3HT (Figure 1). Their optical band gaps are essentially the same. Incomplete photoluminescence quenching was observed in thin films of the 1:1 blend of PHOT and HT-P3HT. PHOT displayed a glass transition at ~269 ºC and decomposed at ~300 ºC according to differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Wide angle X-ray diffraction study showed that PHOT exists in a not highly ordered state in solid films especially in the p-stacking direction. Despite the low LUMO, n-type transporting behavior was not observed for P3HOT). Only p-channel activity was observed in field-effect transistors (FETs) for PHOT. The hole mobility was on the order of 10-4 cm2V-1s-1, which is several orders lower than the highest hole mobility observed for P3HT. The poor charge transporting property is likely due to insufficient p-stacking as demonstrated by the X-ray study. Photovoltaic devices with an active layer of 1:1 blend of PHOT and PC71BM had a power conversion efficiency of ~0.5%. Although the effort did not result in a good n-type polymer, it lead us to realize that efficient p-stacking even in the current best systems is precarious. Our future effort will put a strong emphasis on this issue.
Impact to Career and Future Research Direction
The PRF-ND support allowed the Jia group to enter the area of conducting polymers. The 9,10-diboroanthracene building block can be used for construction of very interesting p-systems. Collaboration with Dr. Meador at NASA-Glenn Research Center has been established based on the mutual interest in anthracene-type structures and polyaromatic hydrocarbons in general. Several proposal submissions before the end of this year have been planned concerning the subject.
Copyright © American Chemical Society