AmericanChemicalSociety.com
Reports: B5 44568-B5: Electrodeposition of Metal Alloy and Mixed Oxide Films Using a Single-Precursor Tetranuclear Heteropolymetallic Complexes
Bizuneh Workie, Delaware State University
Electrochemical studies of the tetranuclear Cu/Ni heteropolymetallic (HPM) complexes have shown that the complexes can be used as a single source (unimolecular ) precursor to electrochemically deposit Cu, Cu/Ni alloy, and Cu and Ni oxide films. The process allows controlled net deposition stoichiometry by the metal stoichiometry of the precursor. This controlled unimolecular electrodeposition technique may hold key to producing new types of nano sized particles of metals, alloys and mixed oxide film with specific predefined stoichiometries, and new types of metal alloy and oxide catalysts and coatings. In this work, further electrochemical and surface studies on the Cu/Co heteropolymetallic (HPM) complexes and their electrodeposited films have been conducted. The rotating disk electrode (RDE) hydrodynamic volatmmetry study of the CuCo3 complex demonstrates similar electrochemical results to the previously reported complexes of Cu3Co and Cu2Co2 HPM complexes1. We also conducted scanning electron microscopy (SEM)/energy dispersive x-ray spectroscopy (EDS) studies for the electrodeposited films obtained from Cu2Co2 and CuCo3 complexes. Analogous to the result of the deposit of the Cu3Co complex, the EDS studies show that the films obtained from Cu2Co2 and CuCo3 complexes are primarily Cu with trace amounts of Co. The chromatographic purification products of the Cu/Zn HPM transmetallation reactions did not result in solid complexes. This hinders our further attempt to study the complexes.
All the heteropolymetallic complexes were synthesized in methylene chloride by means of transmetallation reaction and the by product of the reaction was separated from the complexes by gel permeation chromatography. 2-3 The reactants used for the transmetallation reactions were synthesized according to references 4, 5, and 6.
All cyclic (CV) and RDE voltammetry studies were carried using PAR Model 263A Potentiostat – Galvanostat (PerkinElmer) with three electrodes system consisting of Pt working, Pt wire counter, and Ag/(0.01 M) silver hexafluorophosphate (AgPF6)-CH3CN reference electrodes. The Pt working electrodes for CV and RDE experiments were 3.0 mm diameter (CH instrument Co.) and 1.1 cm (Pine Instrument Co.) disks, respectively. The Pt working electrodes were polished with 0.05 mm alumina (Buehler), washed with deionized water, sonicated for about 5 minutes, and dried before use. All experiments were carried out using 1.0 mM solutions of the complexes with 0.20 M tetrabutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte in dimethylsulfoxide (DMSO). The solutions were bubbled with N2 prior to the electrochemical measurements and blanketed with N2 while conducting the experiments.
SEM study was performed using an AMRAY 1810T microscope with tungsten filament running at 20 kV. The EDS system used was an Oxford Instruments Pentafet detector with a SATW window for analysis down to boron. The energy range typically examined was 1 to 15 kV with a resolution of 134 eV at 5 keV.
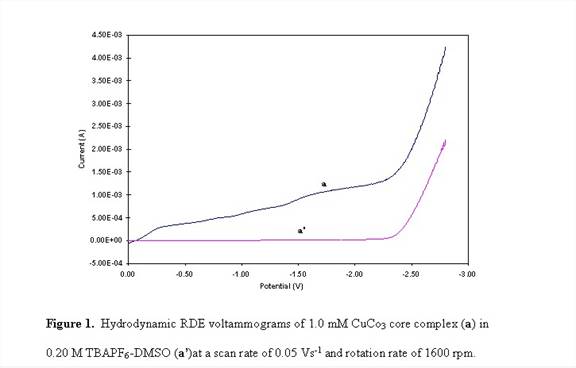
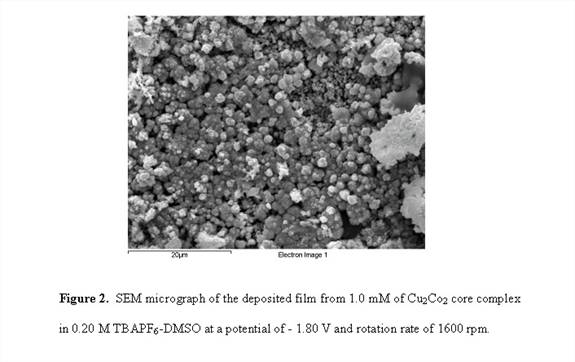
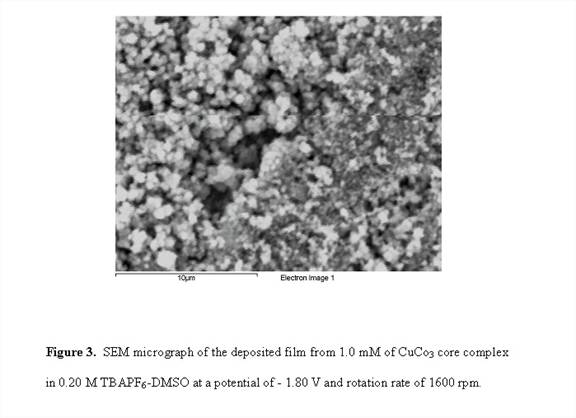
RDE voltammograms of CuCo3 complex shown in Figure 1 exhibit the appearance of two plateau regions. Similar to the Cu3Co and Cu2Co2 complexes, CuCo3 resulted in an electrodeposited film in the potential range of the second plateau. One-hour potentiostatic hydrodynamic RDE electrodeposition of the Cu2Co2 and CuCo3 complexes at potential of - 1.80 V and a rotations rate of 1600 rpm produced a continuous and well-adhered film. The SEM micrograph of the resulting deposits of Cu 2Co and CuCo3 films are shown in Figures 2 and 3. EDS studies of the electrodeposited film of Cu2Co2 complex reveals ~99% Cu and 1% Co along with other trace elements consistent with the electrodeposition solution. For CuCo3, the EDS result shows ~ 94% Cu and 6% Co.
In the initial redox potential regions, the Cu/Co HPM complexes underwent reduction oxidation steps with no electrodeposited film. At higher negative potentials the complexes gave electrodeposited films that are primarily Cu with trace amount of Co. Further works will be conducted to control the Cu/Co atomic ratio of the deposit films by the metal stoichiometry of the complexes and extend the project to other types of heteropolymetallic complexes.
References 1. Workie, B, ACS-PRF 53rd Annual Research Report, 2008, 44568-B5.
2. Davies, G.; El-Sayed, M. A.; El-Toukhy, A., Chem. Soc. Rev., 1992, 101.
3. Caulton, K. G.; Davies, G.; Holt, E. M., Polyhedron, 1990, 9, 2319.
4. Dieck, H. T., Inorg. Chim. Acta, 1973, 7, 397.
5. El-Toukhy, A.; El-Essawi, M.; Tawfik, M.; El-Sayed, L.; Iskander, M. F., Trans. Met. Chem., 1982, 7, 158.
6. El-Sayed, L.; El-Toukhy, A.;Iskander, M. F., Trans. Met. Chem., 1979, 4, 300.
Copyright © American Chemical Society