AmericanChemicalSociety.com
Reports: G5 47849-G5: Structure - Property Relation of Monolayer Catalysts Obtained by Galvanic Displacement of Underpotentially Deposited Monolayers
Stanko Brankovic, University of Houston
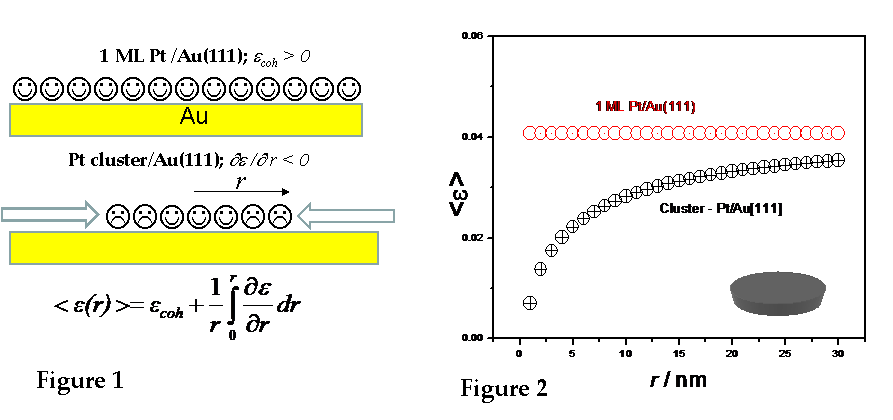
The model system for our study was Pt submonolayer (SML) on Au(111). Due to the epitaxial misfit between Pt and Au, the expected level of coherent strain in full Pt monolayer (ML) on Au(111) is +4%. According to predictions of Nørskov et al[1], this should give the positive rise to the d-band center of the Pt with respect to Fermi level resulting in increased reactivity and activity of Pt ML. In Pt SML configuration i.e. the nanoclusters configuration, the effect of the compressive strain gradient originating from the unpaired bonding of the Pt atoms in the perimeter (edge tension) is expected to have significant effect on the average strain level in Pt nanoclusters, Figure 1. The calculations using continuum model developed by Kern et al[2] have been performed to evaluate this effect. They suggest that average strain in Pt nanoclusters is significantly different from the coherent strain set by epitaxial misfit between Pt and Au. The effect is particularly large for Pt nanoclusters smaller than 5 nm2 while for the Pt nanoclusters larger than 25 nm2 the effect becomes negligible, Figure 2.
In
order to verify these theoretical considerations, the study of the kinetics of
H2 oxidation on Pt SML on Au(111) with different mean cluster size was performed. The Pt SMLs were produced by galvanic displacement of Cu
underpotentially deposited (UPD) ML on Au(111)
substrate[3]. The UPD ML of Cu/Au(111)
was formed from 0.01 M Cu2+ + 0.1 M HClO4 solution. The
galvanic displacement of Cu UPD ML by Pt was carried out from 10-3 PtCl62-
+ 0.1 M HClO4 solution. The coverage of displaced Cu UPD ML on Au(111) has been varied from 0 to 100%, and corresponding
coverage of the Pt SML deposit was investigated by ex-situ scanning tunneling
microscope (STM). In all experiments, more than 95%
of Pt deposit was found to be 2-dimensional, indicating a true SML catalyst configuration. The custom image processing
algorithm based on Otsu's method for threshold determination has been developed
in our lab to process numerous STM images from each deposition
experiment. From these analysis the mean Pt cluster size and Pt coverage is
determined for each deposition experiment. The activity of such prepared Pt SMLs was studied in terms of the calculated representative average
strain in Pt clusters with particular mean size, and resulting exchange current
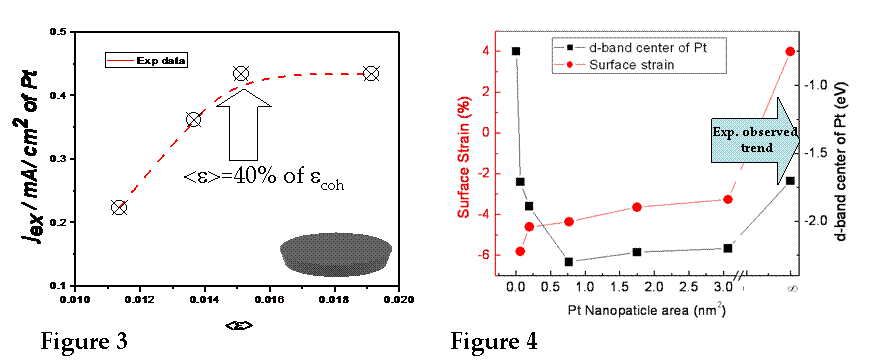
density for H2 oxidation reaction normalized per Pt SML area. The results are shown in Figure 3. As one could
see, the activity of Pt nanoclusters is increasing linearly with the the increase of the tensile (positive) strain in the
nanoclusters. Once the tensile strain in nanoclusters reaches ~40% of the
coherency strain (ecoh) for the full Pt ML
on Au(111), no significant change in the activity of
Pt nanoclusters is observed. In
collaboration with Dr. Liu from BNL, the DFT calculations were performed for Pt/Au(111)
ML and SML configuration to evaluate the surface
strain and d-band center of Pt nanoclusters as a function of their size. The
results are shown in Figure 4. For the ultimately small Pt nanoclusters (<1 nm2) the d-bend shift is significant and
positive, indicating strong effect of under-coordination on reactivity of Pt
clusters. These clusters are practically unstable which renders their
experimental insignificance. However, for the clusters >1 nm2 the increase in d-bend center is closely
following the increase in the tensile strain of Pt clusters, which is the trend
already observed in our experimental measurements, Figure 3. Thus, full
agreement between our experimental data and DFT
predictions for Pt SML on Au(111)
has been demonstrated. We believe that
these findings will improve our understanding of the ML and SML
catalysts desing leading to the control of catalyst ML properties at an atomic
scale. The
topic of this research has been the base for Ph.D. work of one graduate student
which embodied both experimental and theoretical work in the field of catalysis
and electrochemical thin film growth. During the course of this research, the
graduate and undergraduate students of my group were able to get training in
multidisciplinary fields of material science and chemistry, as well as in
diverse experimental techniques such as STM, AFM, RD, RRD, image processing, LSV, CV, etc
This program has served also as base to
establish collaborative work between Dr. Brankovic's group at Univ. of Houston
and Dr. Ping Liu's group at BNL and Dr. Manfred
Buck's group at Univ. of Sent Andres, UK.
[1] M. Mavrakakis, B. Hammar, and J.K. Nørskov, Effect of Strain on the Reactivity of Metal Surfaces, Phys. Rev. Lett., 81, 2819 (1998).
[2] R. Kern, and P. Muller, Elastic Relaxation in Epitaxially Strained Deposits, Surface Science, 392, 103 (1997).
[3] S. R. Brankovic, J. X. Wang and R.R. Adzic, Metal Monolayer Deposition by Replacement of Metal Adlayers on Electrode Surfaces, Surface Science, 474, L173 (2001).
Copyright © American Chemical Society