AmericanChemicalSociety.com
Reports: DNI1 48829-DNI1: Transition-Metal Catalyzed Direct Reductive Coupling of Organic Halides With Electrophiles
Daniel J. Weix, PhD, University of Rochester
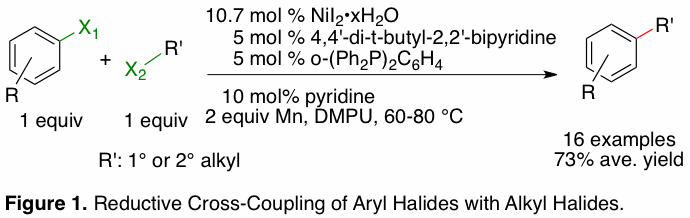
In the past year we have made significant progress towards the development of a general strategy for the coupling of organic halides with other carbon electrophiles. Initial successes include a general reductive cross-coupling of alkyl halides with aryl halides that is catalyzed by nickel (Figure 1).
Notably,
the reaction is tolerant of even sensitive functional groups, such as
unprotected ketones and hydroxyls. While we do not yet have a complete
mechanistic understanding of the origin of the high cross-selectivity observed,
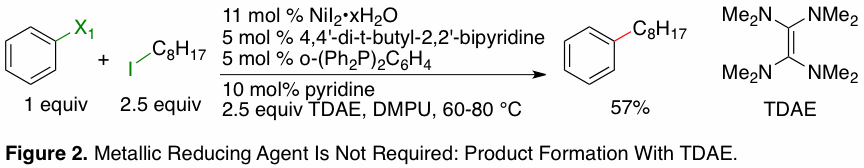
we have shown that the reaction likely does
not proceed by the in situ formation of organomanganese
reagents (Figure 2). In a key experiment, tetrakis(dimethylamino)ethylene, an organic reductant similar in
potential to zinc, was shown to produce product in good chemical yield and with
similar selectivity to reactions that used metallic reductants
(Zn and Mn powder). We
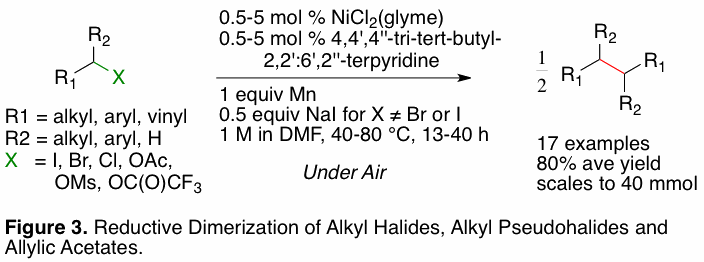
have also become interested in Csp3-Csp3 bond formation
by reductive coupling. As an initial foray into this area, we developed a
method for dimerizing alkyl halides, alkyl pseudohalides, and allylic acetates under very mild
conditions (Figure 3). Prior to this work, no protocol existed for the dimerization of functionalized alkyl halides less reactive
than alkyl iodides. Follow-on studies will seek to expand this work to include
the cross-coupling of two alkyl halides. Collectively,
these preliminary studies have revealed the beginnings of a series of general
procedures for coupling organic halides with a variety of carbon electrophiles.
Future work will focus on expanding the scope of the electrophiles that can be
coupled with organic halides and identifying the origins of the observed
selectivity.
Copyright © American Chemical Society