AmericanChemicalSociety.com
Reports: GB3 47823-GB3: Disruption of Metal-Metal Interactions and Extended Linear Chains by Organic Solvent Molecules
Zerihun Assefa, North Carolina A&T State University
1. Introduction
The importance of metal containing polymers for new materials is quite extensive and Au(I) and Pt(II) based compounds are of particular importance due to their interesting physical and chemical properties.1-3 In such compounds, aurophilic (Au-Au) and platinophilic (Pt-Pt) interactions are prominent and recognized as a major force determining structural dimensionality and properties.4,5 The extended metal-metal interactions, as reported in numerous examples, proceeds in ligand unsupported fashion. Pt(II), Au(I), and Ag(I) in particular are known to exhibit this behavior, where the weak metal-metal interaction leads to aggregation and formation of dimeric and extended chain systems4,5. Earlier studies on Pt(II) tetra cyano complexes demonstrated the presence of pseudo-1-D columnar chains6 of planar anions stacked parallel to one another. The fund from the PRF program has allowed us to continue investigating heterobimetallic systems where factors that influence the extent of metal-metal interactions have been the focus of our studies.
2. Results
a) We are actively pursuing the synthetic and spectroscopic properties of metal containing polymeric materials. The significance of Pt-Pt interactions that occur between adjacent Pt tetracyano anions is apparent as these materials exhibit highly tunable spectral properties. The Pt-Pt distances and the associated spectroscopic properties can be tuned by chemical and physical variations including variation of counter cation, applied pressure, temperature and solvent. A continued research effort involves cyanide-bridged heterometallic systems that are prepared by assembling cyanometallates, e.g. [Pt(CN)4]2, and transition metals or lanthanide ions. Such materials are found to exhibit fascinating structural, birefringent, luminescent, and sensing properties7-9. Our recent progress in these area involved incorporation of Au(I) units in various ratios into the lanthanide containing Pt(II) systems. Heterometallic cluster units consisting of Pt-Au and Pt-Pt interactions in close proximity were observed. Apart from tetra and hexanuclear metallic arrangements, the materials exhibit interesting spectroscopic behaviors, where exciplex and excimer emissions dominate the spectral profile.
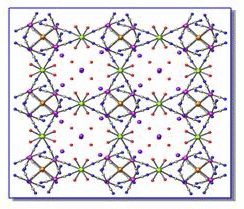 Similar
behaving compounds are now characterized crystallographically that exhibit
channel systems and have the potential to absorb VOCs in the pores. The
compound shown below is the structure of the K2[Tb(H2O)4(Pt(CN)4)2]Au(CN)2·2H2O system that consists
of two-dimensional layers formed by the linkage of the Tb3+ cations through
cis-bridging tetracyanoplatinate anions to four additional Tb3+
cations. This arrangement results in a larger pore of 24-member ring with pores
that can be occupied by ions and/or VOC's. The structure displays Au-Au and
Au-Pt interactions which provide the source of luminescence in the system.
Similar
behaving compounds are now characterized crystallographically that exhibit
channel systems and have the potential to absorb VOCs in the pores. The
compound shown below is the structure of the K2[Tb(H2O)4(Pt(CN)4)2]Au(CN)2·2H2O system that consists
of two-dimensional layers formed by the linkage of the Tb3+ cations through
cis-bridging tetracyanoplatinate anions to four additional Tb3+
cations. This arrangement results in a larger pore of 24-member ring with pores
that can be occupied by ions and/or VOC's. The structure displays Au-Au and
Au-Pt interactions which provide the source of luminescence in the system.
Similarly other structures corresponding to [Tb(C10N2H8)(H2O)4(Pt(CN)4)(Au(CN)2)] system were also found to exhibit pore features. These systems have dual advantages in the detection of VOCs. On one hand they are among the most prevalent form of extended linear chain (ELC) compounds used in VOC sensing10. On the other hand, they exhibit a co-planar-arrangement with a channel system within the lattice that can hold VOC's of interest. The channel system can provide a pathway for organic vapors to easily in and out of the lattice.
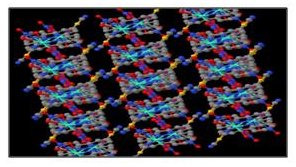 b) In a related area the PRF fund has also allowed
us to initiate a collaborative work involving dual-donor sensitization and
excited state dynamics with the Sykora's group at the University of South
Alabama and an international collaborative work with a Japanese scientist
(Tsuyoshi Yayita). The Au(I) and Pt(II) based coordination polymers that
exhibit vapoluminescent properties have been targeted since they have unique
features. Their square planar units stack one unit on top of the other forming
a one dimensional columnar arrangement with nominal metal-metal interactions. In
several instances the materials exhibit a hollow low-dimensional structure
which has been our main focus for the last several years since the hollow
feature allows VOC molecules to line channels along a particular
crystallographic axis and provide a possible route for diffusion of vapors in
and out of the host lattice. Fundamental understanding of emission enhancement
through dual donor sensitization is progressing. This is a new area that our
group initiated in collaboration with Sykora's group at the University of South Alabama. We believe the use of multiple donors for systematic design of
luminescent lanthanide probes is an area that needs to be developed since an
understanding of the basic mechanisms of emission enhancement through a
cooperative effect may lead to a rational design of materials useful for
various applications. In our preliminary work in this area we have chosen two
donor systems that both absorb in the UV region and undergo energy transfer
processes to selected lanthanide ions. The chosen systems involve the tetracyanoplatinate
(TCP) and the terpyridine
ligand directly coordinated to the lanthanide ion as donors.
b) In a related area the PRF fund has also allowed
us to initiate a collaborative work involving dual-donor sensitization and
excited state dynamics with the Sykora's group at the University of South
Alabama and an international collaborative work with a Japanese scientist
(Tsuyoshi Yayita). The Au(I) and Pt(II) based coordination polymers that
exhibit vapoluminescent properties have been targeted since they have unique
features. Their square planar units stack one unit on top of the other forming
a one dimensional columnar arrangement with nominal metal-metal interactions. In
several instances the materials exhibit a hollow low-dimensional structure
which has been our main focus for the last several years since the hollow
feature allows VOC molecules to line channels along a particular
crystallographic axis and provide a possible route for diffusion of vapors in
and out of the host lattice. Fundamental understanding of emission enhancement
through dual donor sensitization is progressing. This is a new area that our
group initiated in collaboration with Sykora's group at the University of South Alabama. We believe the use of multiple donors for systematic design of
luminescent lanthanide probes is an area that needs to be developed since an
understanding of the basic mechanisms of emission enhancement through a
cooperative effect may lead to a rational design of materials useful for
various applications. In our preliminary work in this area we have chosen two
donor systems that both absorb in the UV region and undergo energy transfer
processes to selected lanthanide ions. The chosen systems involve the tetracyanoplatinate
(TCP) and the terpyridine
ligand directly coordinated to the lanthanide ion as donors.
References
- Forward, J. M.; Fackler, Jr., J. P.; Assefa, Z. in: Roundhill, D. M.; Fackler, Jr., J. P. (Eds.), Optoelectronic Properties of Inorganic Compounds, Plenum Press, New York, 1999, pp. 195229.
- Yam, V. W.-W.; Wong, K. M.-C.; Zhu, N. J. Am. Chem. Soc. 2002, 124, 6506.
- Katz, M. J.; Kaluarachi, H.; Batchelor, R. J.; Bokov, A. A.; Ye, Z.-G.; Leznoff, D. B. Angew. Chem., Int. Ed. 2007, 46, 8804.
- Leznoff, D. B.; Xue, B.-Y.; Patrick, B. O.; Sanchez, V.; Thompson, R. C. Chem. Commun. 2001, 259.
- Fernández, E. J.; Laguan, A.; López-de-Luzuriaga, J. M. Dalton Trans. 2007, 1969.
- Gliemann, G.; Yersin, H. Struct. Bond. 1985, 62, 87 & refs. therein.
- Katz, M. J.; Kaluarachi, H.; Batchelor, R. J.; Bokov, A. A.; Ye, Z.-G.; Leznoff, D. B. Angew. Chem., Int. Ed. 2007, 46, 8804.
- Leznoff, D. B.; Xue, B.-Y.; Patrick, B. O.; Sanchez, V.; Thompson, R. C. Chem. Commun. 2001, 259.
- Katz, M. J.; Ramnial, T.; Yu, H.-Z.; Leznoff, D. B. J. Am. Chem. Soc. 2008, 130, 10662.
- Drew, S. M.; Smith, L. I.; McGee, K. A.; Mann, K. R. A platinum(II) extended linear chain material that selectively uptakes benzene. Chem. Mater. 2009, 21, 3117-3124.
Copyright © American Chemical Society

