AmericanChemicalSociety.com
Reports: DNI10 48810-DNI10: New Mechanisms of Hydrogen Storage from Chemical Frustration
Peihong Zhang, PhD, State University of New York at Buffalo
Hydrogen fuel cells are attractive for their very high efficiency. Beyond the technology of the cells themselves, these systems present two major challenges: economic large-scale hydrogen production and practical small-scale on-board hydrogen storage. The storage problem in particular demands fundamentally new transformative science and engineering.
We have been investigating a novel layered solid boron hydride which has a gravimetric capacity of 8 wt% and show great potential for hydrogen storage. Pure hexagonal boron layer, although not stable alone due to electron deficiency, can be stabilized by intercalating electron donors such as Mg and Al to form MgB2 and AlB2. In this work, we propose that the electron donor can also be hydrogen atoms through the formation of 3c2e bridge bonds [Fig. 1(a)].
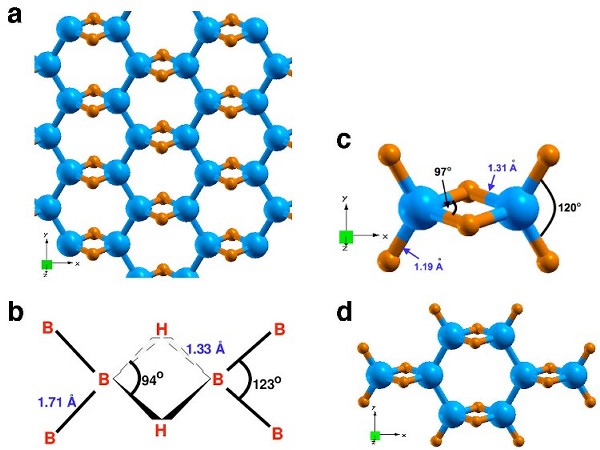 |
| Fig. 1. (a) Structure of single-layer B2H2. (b) The corresponding bond angles and bond lengths. (c) The structure of diborane. (d) The structure of octaborane. Boron is blue and hydrogen is orange. |
The boron atoms in B2H2 structure form 3c2e bonds with bridging hydrogen atoms and connect to other in-plane boron atoms via a conventional sp2 hybridization. These weakly bonded hydrogens are crucial to stabilize the boron network and are desirable for the release of hydrogen at practical conditions. The extended boron framework in this solid boron-hydride structure is quite distinct from a collection of individual diborane molecules [Fig. 1(c)]. Preservation of the backbone boron structure over cycling (by means of kinetic barriers against framework decomposition) would be extremely advantageous for regeneration as compared to traditional boron hydrides, i.e., boranes.
Structural properties and energetics of single-layer B2H2
We have carried out detailed studies on the electronic, structural, and thermodynamic properties of the B2H2 structure using density functional theory based electronic structure methods. The structure has a gravimetric capacity of 8 wt% H2. The calculated bond angles and bond lengths of B2H2 [Fig. 1 (b)] are close to those in diborane [Fig. 1 (c)].
Electronic structure of B2H2
The band structure of the single-layer B2H2 is shown in Fig. 2 (a). The total density of states (DOS) and the projected density of states (PDOS) for boron and hydrogen orbitals are shown in Fig. 2 (b) and (c). It is interesting to compare the band structure of planar B2H2 to that of MgB2. Like MgB2, the system is metallic. However, unlike that of MgB2, the degeneracy of the p-σ bands at the Γ-point in MgB2 is now removed due to lowering of the symmetry from D6h to D2h. As a result, only one of the $p-σ bands crosses the Fermi level and the hole pocket near the Γ-point comes from the upper p-σ band.
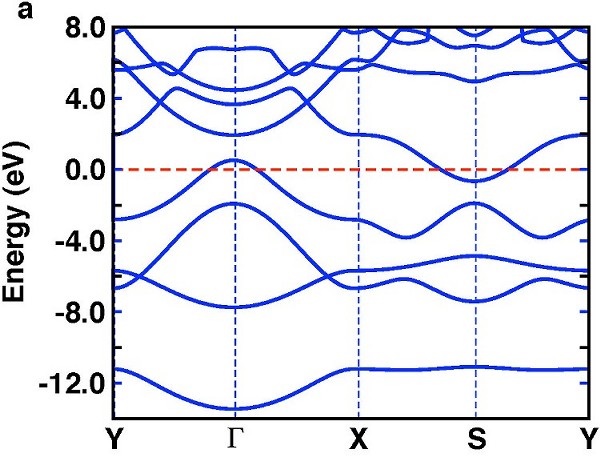 |
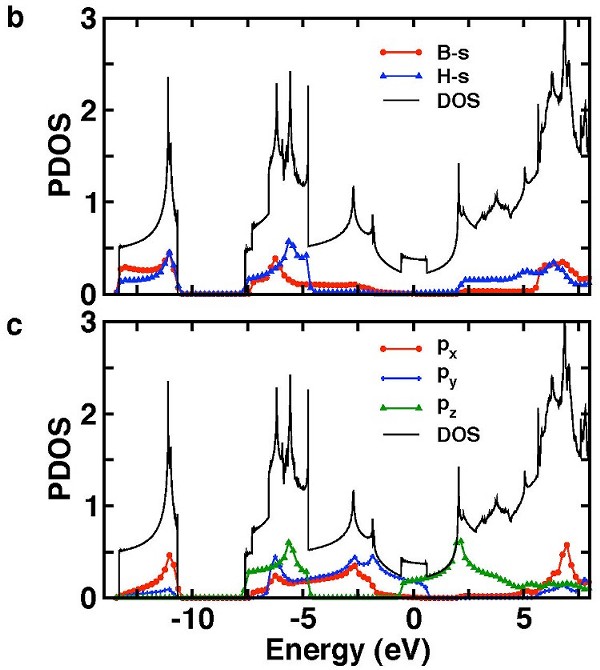 |
| Fig. 2. (a) Band structure of a single-layer of B2H2. (b) Projected density of states (PDOS) and total density of states (DOS) for B 2s and H 1s. (c) PDOS and DOS for B-2px, B-2py and B-2pz. |
Phonon dispersion, dynamical and thermodynamical stability
To investigate the dynamical stability of the proposed structure, we carry out phonon calculations using density functional perturbation theory~. The calculated phonon dispersion (shown in Fig. 3) reveals no soft phonon modes, thus verifying the dynamical stability of the B2H2 structure. The nine optical phonon modes can be classified using group theory analysis: Γvib= Ag1[H-H(z)]+ Ag2[B-B(x)]+ B1g[B-B(y)]+ B2g[H-H(y)] + B3g1[H-H(x)]+ B3g2[B-B(z)]+ B1u[H-B(z)]+ B2u[H-B(x)]+ B3u[H-B(y)].
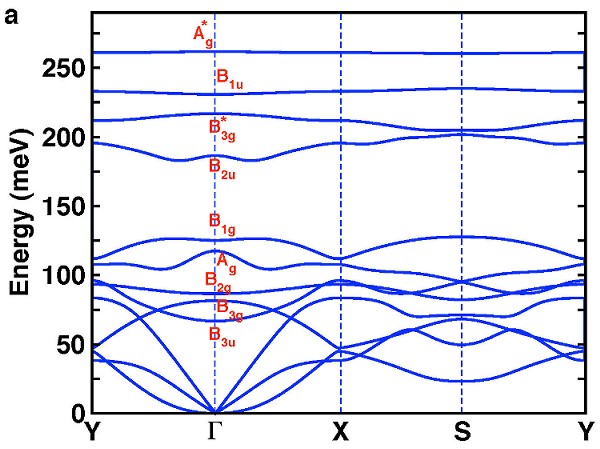 |
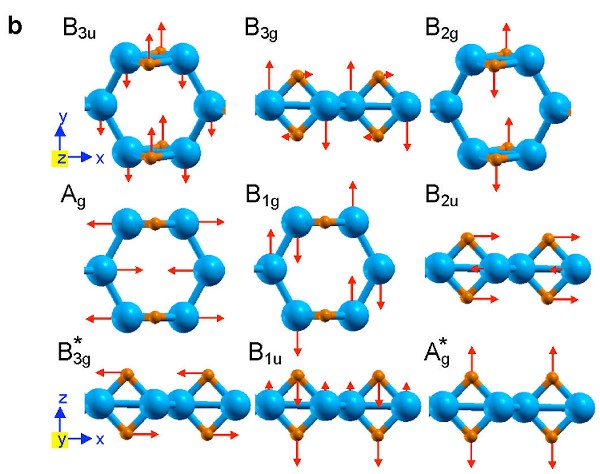 |
| (a) Calculated phonon dispersion for single-layer B2H2. (b) The corresponding phonon polarization vectors of the nine optical phonons at Γ. Boron is blue and hydrogen is orange. |
In addition to the dynamical stability, we also investigated the thermodynamical stability of the B2H2 structure using a long time ab initio molecular dynamics (MD) simulation in a wide temperature range (300 K - 400 K). In this temperature range, we find that the energies of the system remain the same throughout the simulation time, indicating that no phase separation occurs within this temperature range.
Charging assisted hyrdogen release mechanism
Utilizing B2H2 for hydrogen storage requires not only a stable boron framework but also a practical hydrogen release mechanism. A planar hexagonal boron layer is not stable alone due to electron deficiency. If additional electrons are introduced to the B2H2 system (by intercalation of Li or boron self doping), the boron network will be less electron deficient. As a result, the hydrogen bridge bonds should weaken with charging. The hexagonal boron backbone structure, on the other hand, should remain stable with charging. Therefore, one may be able to control the strength of the hydrogen bridge bonds by controlling the degree of electron deficiency of the boron network. This, when combined with thermal activation, may facilitate dehydrogenation and/or rehydrogenation. Our results reveal phonon modes involving vibrations of hydrogen are significantly softened by charging. The softening of these hydrogen-related phonons is a result of the weakening of the hydrogen bridge bonds upon charging, which in turn should enhance hydrogen release.
We have also calculated the activation energy of hydrogen release using the nudged elastic band (NEB) method. Our NEB results for the activation energy for the neutral B2H2 is 2.1 eV per H2 molecule. This value is lowed to 0.5 eV with boron self-doping. By changing the self-doping level, the B-H bonding strength can be systematically tuned to match the target bonding strength for practical applications. These results demonstrate the flexibility and tunability of this structure.
In summary, we have predicted a novel layered solid boron hydride structure and investigate its structural, electronic and phonon properties. The phonon dispersion of single-layer B2H2 does not show any soft phonon modes, thus confirming the dynamical stability of the proposed structure. We also investigate the effects of charging on the dynamical properties and hydrogen release kinetics of B2H2. With the presence of extra electrons, the boron network becomes less electron-deficient therefore reducing the strength of the multi-center B-H bonds. These results suggest a charging-assisted hydrogen release mechanism.
Copyright © American Chemical Society

