AmericanChemicalSociety.com
Reports: UR3 49503-UR3: In Situ Thermodynamic Characterization of Supramolecular Assembly Processes that Lead to Discrete Nanosize Structures
Douglas A. Vander Griend, Calvin College
In our quest to study chemical systems with high degrees of organizational, dynamic, and compositional complexity, we have four major scientific results to report. In all of them, the capabilities and limits of the computer program Sivvu have been clarified and extended, respectively.
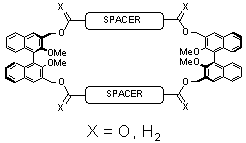
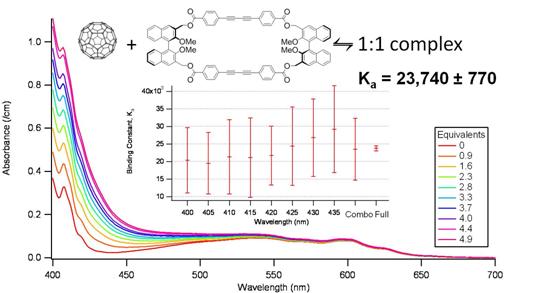
First, C60 has been found to associate with a variety of macrocyclic ligands (Figure 1), exclusively forming 1:1 complexes if the ligand is sufficiently flexible and large. NMR, CD spectroscopy, and computational chemistry help rationalize the shape and binding of the cyclic scaffolds even as subtle modifications in the bridging units lead to drastic changes in conformation. Sivvu has been used to determine the binding constants with a high degree of accuracy: with long spacers and ester linkages, log Ka = 4.37(2); with short spacers and ester linkages, log Ka = 3.498(4); with long spacers and ether linkages, log Ka = 3.509(2). In the last case, the binding event is also detectable by CD spectroscopy, indicating the chirality of the supramolecular complex as a whole. For these systems, it proved to be essential to model the entire curves rather than just a few wavelengths as the signals of all three species overlapped strongly. Figure 2 highlights the accuracy of this modeling approach.
Figure 1: Basic macrocycle structure. Spacer = biphenyl or bis phenylacetylene.
Figure 2: Spectrophotometric titration of C60 with a macrocyclic ligand. Central inset shows the standard error on the binding constant, K, if individual wavelengths are used and even averaged (combo), as compared to the full curve analysis conducted with Sivvu.
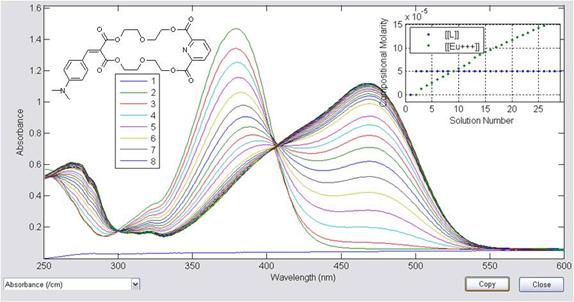
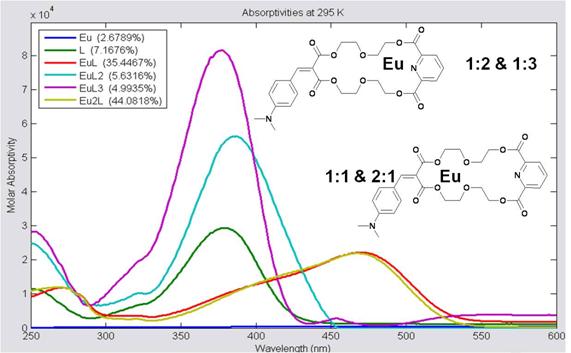
Second, it has been established that the interactions between Eu(III) and a 'push-pull' chromophoric ligand, L, are numerous and varied. Starting with data from a spectrophotometric titration of Eu(OTf)3 into a 5 × 10-5 M solution of the ligand (Figure 3), metal:ligand complexes with stoichiometry of 1:1, 1:2, 1:3, and 2:1 were all identified and characterized (Figure 4).
Figure 3: Spectrophotometric titration data for Eu(OTf)3 and ligand (shown inset upper left). A graph of the composition of each solution is inset on the upper right.
Figure 4: Molar absorptivity values for the six major absorbers in this system. For 1:2 and 1:3 M:L complexes, the Eu(III) likely coordinates to the pyridinal moiety of the ligand. A Eu(III) can also coordinate to the other side of the ligand, and when it does, the absorbance of the ligand red-shifts from 370 nm to 470 nm.
The equilibrium constants for all binding interactions were also determined.
Eu(OTf)3 + L ↔ Eu(L)(OTf)3 logKa1 = 6.25(36)
Eu2(L)(OTf)6 + L ↔ 2Eu(L)(OTf)3 logKa1′ = 5.45(02)
Eu(L) (OTf)3 + L ↔ Eu(L)2(OTf)3 logKa2 = 5.01(4)
Eu(L)2(OTf)3 + L ↔ Eu(L)3(OTf)3 logKa3 = 5.07(6)
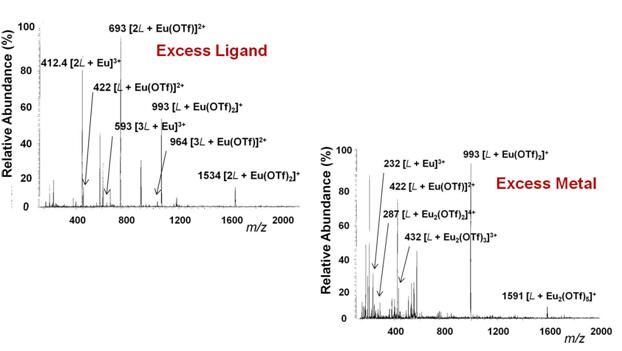
Electrospray mass spectrometry independently confirms all species (Figure 5).
Figure 5: Electrospray mass spectrometry of solutions with excess equivalents of ligand and metal.
Third, the binding affinities for the molecular muscle nanomachine designed by the Flood group at Indiana University were ascertained via spectrophotometric titration at 283 K, 293 K, 303 K and 313 K. This led to the calculation of the entropy and enthalpy terms for various binding events that are integral with the operation of the nanomuscle.
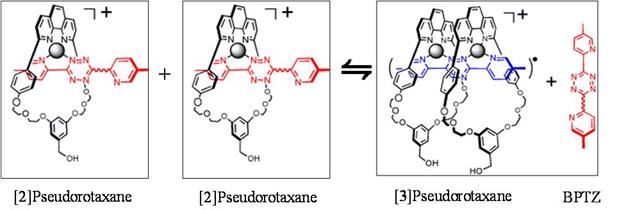
Figure 6: A nanomachine that can potentially function as a molecular muscle. Cu(I) cations bind to a macrocycle and a bipyridyltetrazine to form various pseudorotaxanes, PR.
Macrocyle + Cu(I) ↔ Cu(I)·Macrocyle ΔH0° = 1.69(5) kJ/mol ΔS0° = 94(2) J/mol·K
Cu(I)·Macrocyle + BPTZ ↔ [2]PR ΔH1° = 11.8(1) kJ/mol ΔS1° = 188(27) J/mol·K
Cu(I)·Macrocyle + [2]PR ↔ [3]PR ΔH2° = 5.5(2.6) kJ/mol ΔS2° = 133(8) J/mol·K
2[2]PR ↔ [3]PR + BPTZ ΔH3° = -6.3(2.6) kJ/mol ΔS3° = -55(28) J/mol·K
Finally, a four by four grid complex that assembles via coordinative bonding between Cu(I) and a phenyl-substituted 3,6 - bis (triazole) pyridazine has been studied via spectrophotometric titration. The following metal:ligand complexes have been identified and characterized: 1:2, 2:3, 4:4, 3:2, and 2:1. The sequential binding constants have also been determined. This represents some of the first ever examples of capturing the equilibrium self-assembly process.
Figure 7: The data from the spectrophotometric titration of Cu(CH3CN)4BF4 with ligand can be deconvoluted to show the molar absorptivity values and compositional profiles of seven different absorbers, including the grid complex comprised of 4 Cu(I) centers and 4 connecting ligands.
The impact of the research into equilibrium self-assembly over the last 15 months has been substantial. For the undergraduate students who have worked on this project, it has helped them grow as scientific researchers and communicators. Two of them attended and presented their work at the National ACS meeting this past spring in San Francisco. Both attested that the experience was very exhilarating and formative. They were excited to see how much chemistry is going on the world over. They got to interact with many other chemists including principle investigators at some of the best research institutions. The one who graduated last may is now a graduate student at the University of California, Berkeley in inorganic chemistry. Another plans to go to graduate school in chemistry after Calvin. Her intentions are well-informed as she, along with a Project SEED high school student, was able to spend a week in the lab of my collaborator, Amar Flood, at the University of Bloomington this summer.
As for my career, this research connected me with Prof. Dario Pasini at the University of Pavia with whom I worked for the 5 months of my sabbatical this past fall. Through this opportunity, I was able to make valuable professional connections which have resulted in several publications and an ongoing collaboration. I overhauled the computer program that we rely on heavily and applied our analysis approach to a very wide array of problems, thereby demonstrating its versatility. Additionally, it was at the spring ACS national meeting where I connected with Prof. Mike Ward of Sheffield University, England. The 3-dimensional self-assembly systems that they have developed turned out to be excellent candidates for modeling.
Thank you for supporting this research and making the impact possible.
Copyright © American Chemical Society