AmericanChemicalSociety.com
Reports: DNI1 48864-DNI1: Exploring the B-N/C=C Isosterism in Carbon-Rich Compounds
Shih-Yuan Liu, PhD, University of Oregon
Abstract
The C=C bond unit is a ubiquitous and fundamentally important structural motif in organic chemistry. The substitution of the organic C=C unit with the isosteric inorganic B–N bond in organic molecules can lead to novel hybrid structures with unique properties. The proposed research seeks to illuminate the B–N vs. C=C isosterism in conjugated carbon-rich compounds by investigating the effects of this isosterism in benzene derivatives, i.e., 1,2-azaborines. We have accomplished the synthesis of a versatile intermediate for the construction of 1,2-azaborine-based conjugated scaffolds. We also investigated the synthesis, structural characterization, and optoelectronic properties of BN-Tolan and Bis-BN-Tolan, one of the simplest conjugated systems containing the BN bond pair. BN tolans display absorption and emission properties that are distinct from their carbonaceous analogue, tolan. In addition, we discovered that Bis-BN-Tolan exhibits unique N–H-(CC) hydrogen bonding in the solid state.
Specific Aims
This proposed research seeks to study the B–N vs. C=C isosterism in conjugated structures incorporating the 1,2-azaborine core (Scheme 1). Specifically, we plan:
1) to develop a synthesis of a versatile 1,2-azaborine precursor amenable to substitution at boron, nitrogen, and carbon positions.
2) to construct a series of phenylacetylene and dehydrobenzoannulene structures with B–N substitution at various positions.
3) to develop a program to characterize the structural, photophysical, and redox property of the newly prepared compounds.
4) to explore the fundamental chemical reactivity of these carbon-rich scaffolds, such as the Bergman cyclization, to generate more complex structures that will permit a thorough examination of the effects of B–N substitution.
Accomplishments
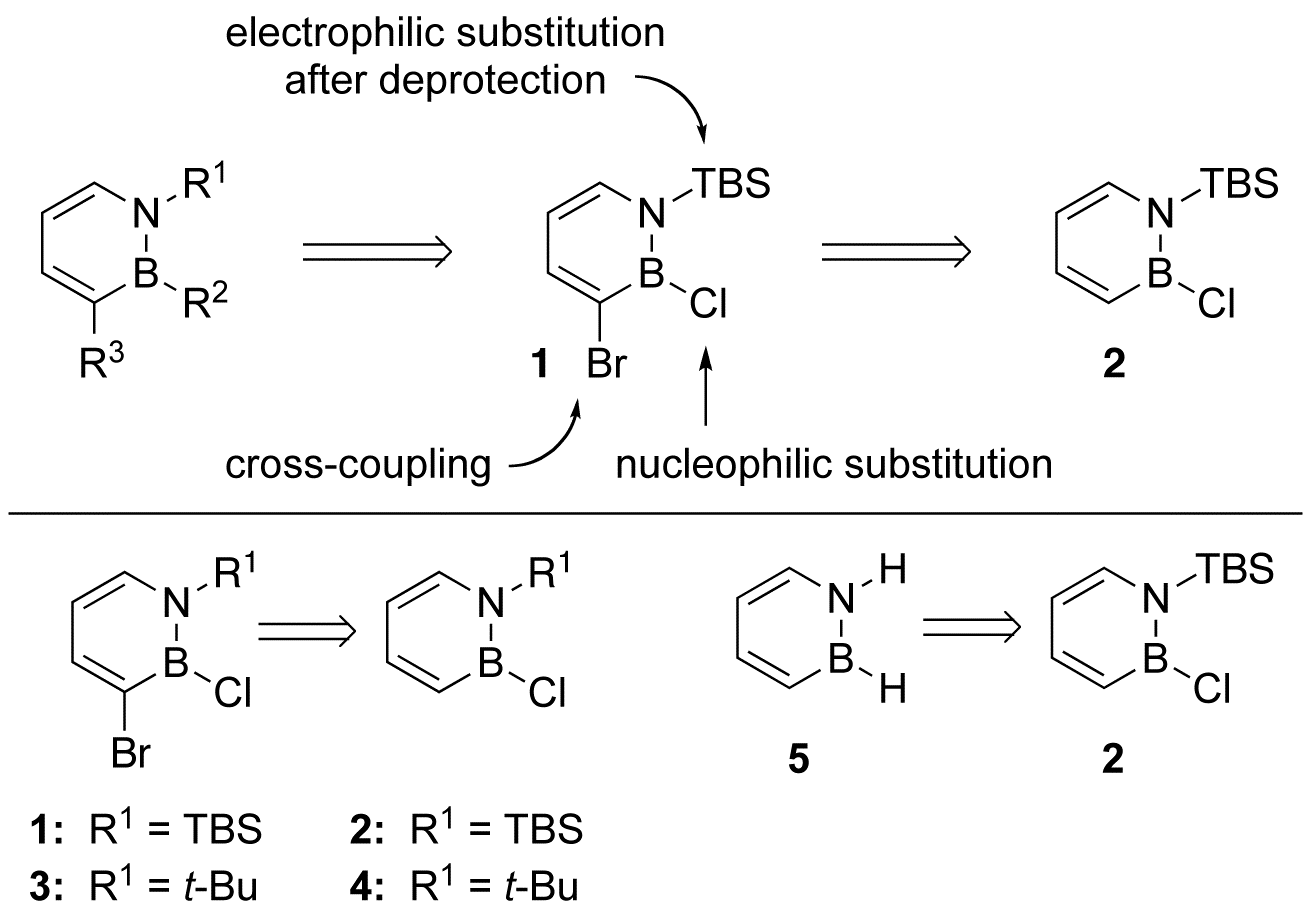
The proposed research is strongly dependent on the successful synthesis of the universal precursor 1 bearing three orthogonal functional features (Scheme 2, top). The strong dependence of the success of the proposed research on a single crucial target compound has been pointed out by several reviewers of the proposal. Cognizant of the importance of compound 1, we pursued its synthesis prior to ACS-PRF funding. We determined that treatment of 1,2-azaborine 2 with elemental bromine gave the desired compound 1 in 61% isolated yield (Scheme 2, bottom). Compound 1 has been characterized by 1H, 13C, 11B NMR, IR, and high-resolution mass spectroscopy. The related compound 3 has been prepared in the same manner from precursor 4, the procedure for which has been published in Mol. Biosyst. 2009, 5, 1303-1305. We also determined that the N-TBS can be subsequently removed, which ultimately led to the first successful isolation and characterization of the parent 1,2-Dihydro-1,2-azaborine 5 (Scheme 2, bottom, Angew. Chem. Int. Ed. 2009, 48, 973-977).
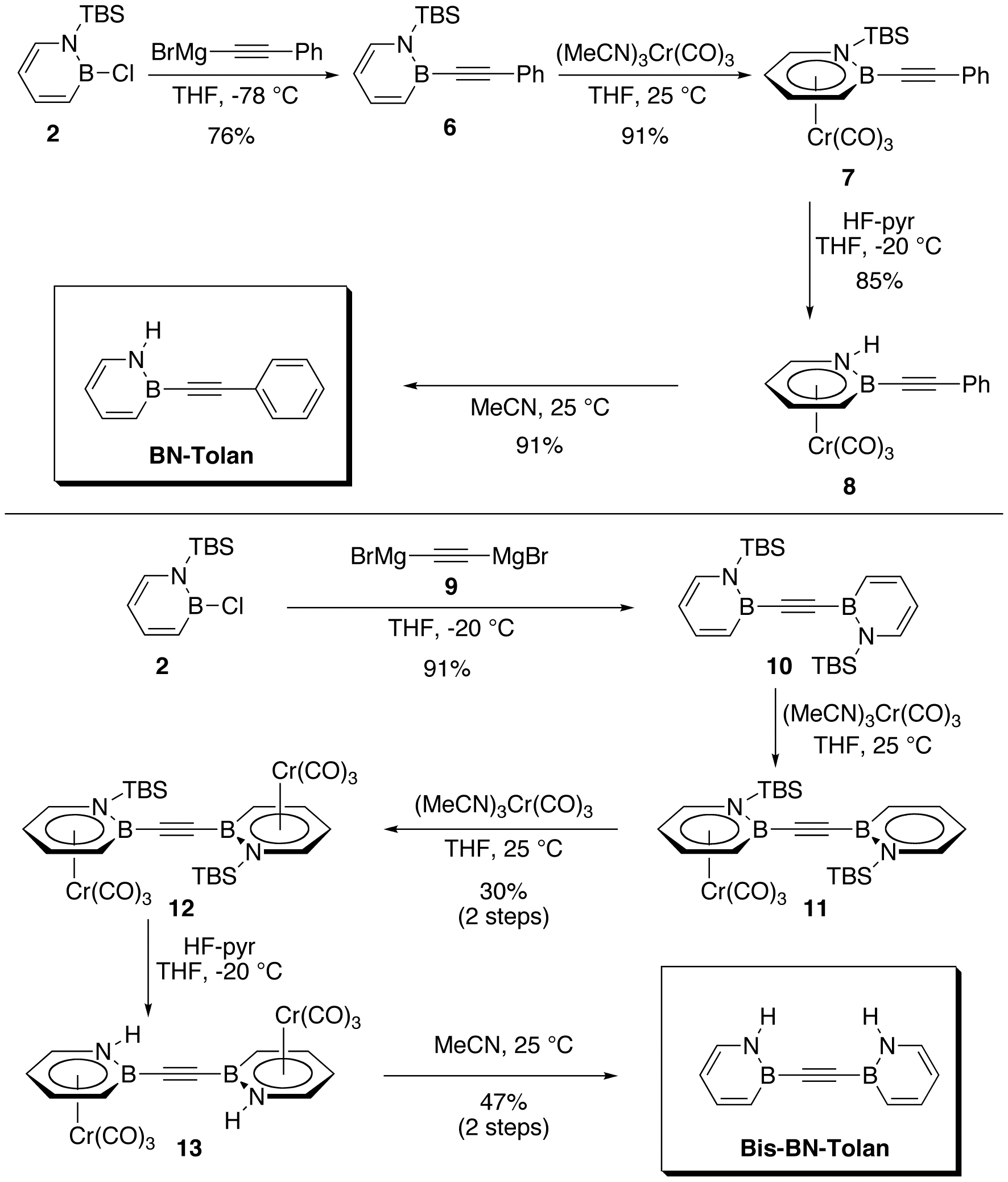
Having versatile synthetic precursors 1 and 2 available, and with the help of ACS-PRF funding, we have chosen BN-substituted diphenylacetylene (tolan) as our initial conjugated system to investigate because of its structural simplicity and its significance in materials science. Diphenylacetylene serves as an important building block in liquid crystals and is the fundamental sub-unit in the structure of the carbon allotrope graphyne.
Scheme 3 illustrates the synthesis of BN-Tolan and Bis-BN-Tolan. Nucleophilic substitution of B-Cl 1,2-azaborine precursor 2 with phenylethynyl magnesium bromide furnished N-TBS protected 6 in 76% isolated yield. The reaction of 6 with (MeCN)3Cr(CO)3 gave piano-stool complex 7 in 91% yield. Deprotection of the N-TBS group with HF¥pyridine afforded the chromium tricarbonyl complex 8. Dissolution of 8 in MeCN, followed by silica gel chromatography gave BN Tolan in 91% yield.
The synthesis of Bis-BN-Tolan was accomplished in a similar manner. In situ generation of Grignard reagent 9 and subsequent reaction with 1,2-azaborine precursor 2 produced 10 in good yield. The complexation of 10 with Cr(0) proved more challenging, ultimately furnishing complex 12 in 30% isolated yield via complex 11. Removal of the N-TBS group with HF¥pyridine afforded 13 as a highly-insoluble orange solid, which was dissolved directly in MeCN to yield Bis-BN-Tolan in 47% isolated yield over two steps.
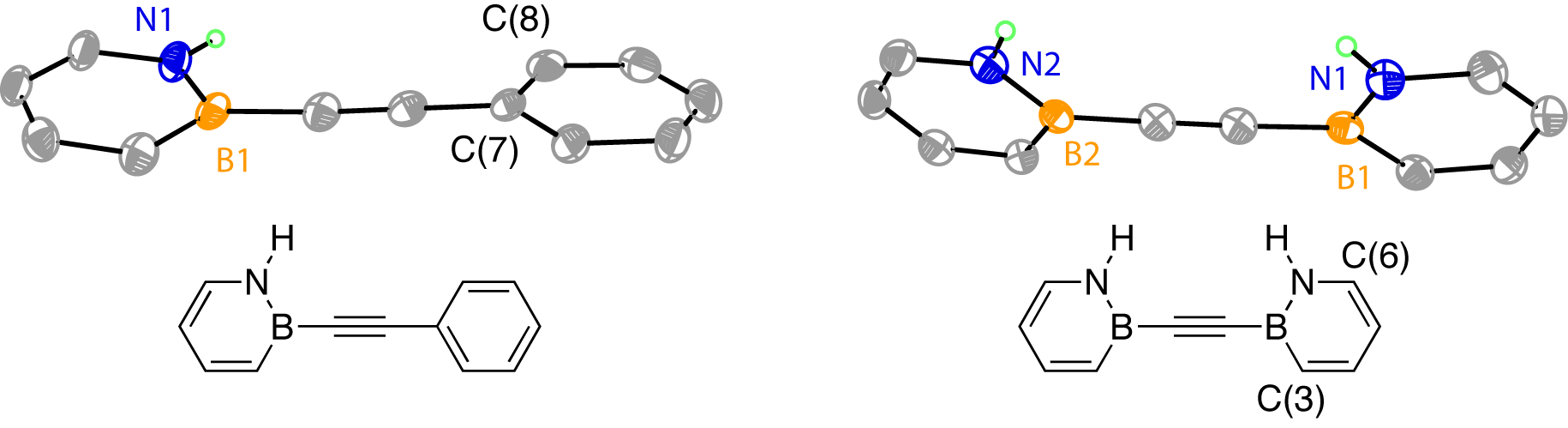
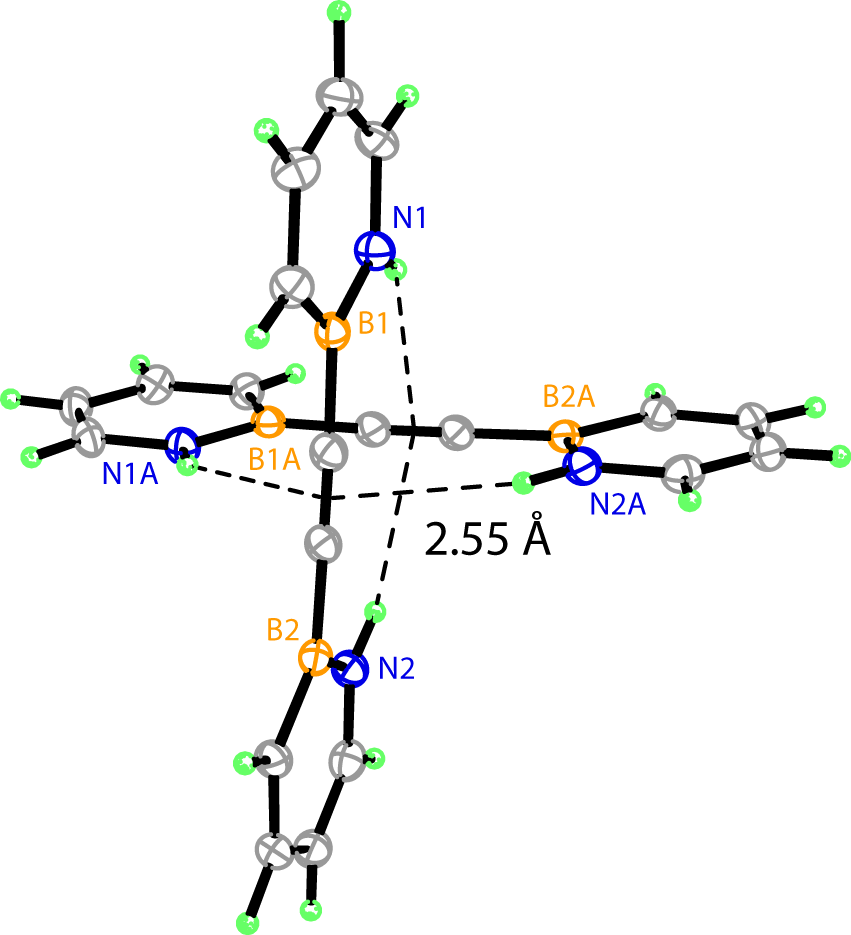
We obtained the X-ray crystal structures of BN-Tolan and Bis-BN-Tolan, thus unambiguously establishing their structural identities (Figure 1). The crystal packing of Bis-BN-Tolan reveals noteworthy features. There appears to be a short contact between one of the N–H groups and the alkyne bridge of adjacent Bis-BN-Tolan molecules (N–H-(CC) = 2.55 , angle N–H-(CC) = 159°) (Figure 2). Hydrogen-bonding between an N–H donor and alkyne acceptor is relatively rare. For the few known examples, the mean N–H-(CC) bond distance is 2.36, and the mean N–H–(CC) angle is 164.3°. The formally isoelectronic relationship between 1,2-azaborine and the phenyl ring allows the N–H-(CC) interaction in Bis-BN-Tolan to be considered as a mimic of C–H-(CC) interaction. Solid-state C–H-(CC) distances (mean distance = 2.854(7) ) are generally longer than those observed for N–H-(CC) and deviate more greatly from 180° (mean angle C–H–(CC) = 148.1(9)°). Thus the observed short N–H-(CC) interaction in Bis-BN-Tolan could be considered intermediary between typical C–H-(CC) and N–H-(CC) hydrogen bonds. Thin-film infra-red spectroscopy indicates a slightly lower stretching frequency of the N–H bond in Bis-BN-Tolan (3370 cm–1) compared to the parent compound, 1,2-Dihydro-1,2-azaborine (NH = 3400 cm-1).
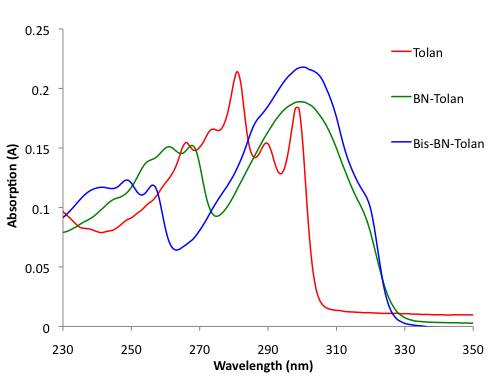
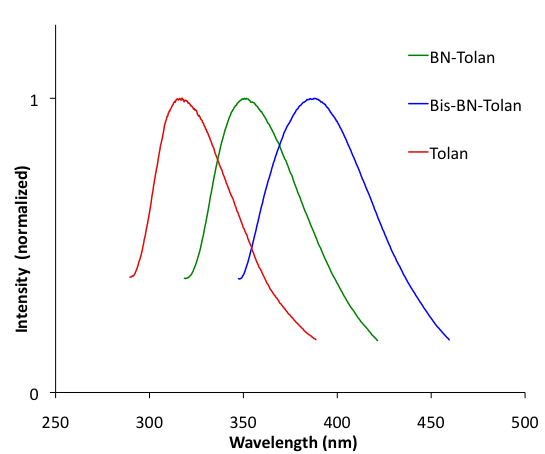
We also investigated the optoelectronic properties of BN-Tolan, Bis-BN-Tolan, and tolan. Both BN-Tolan and Bis-BN-Tolan have an absorption at 299 nm, which is broadened significantly relative to the corresponding absorption in tolan (Figure 3). As a result, BN-Tolan and Bis-BN-Tolan absorb out to 325 nm. Broadening of the lowest energy absorption bands in 1,2-azaborines has been attributed to lower symmetry in the heterocycle versus the phenyl ring. Figure 4 illustrates the emission spectra of BN-Tolan, Bis-BN-Tolan, and tolan in THF. The emission of BN-Tolan (350 nm, FFL = 0.012) is bathochromically shifted relative to tolan (317 nm, FFL = 0.007), while the emission of Bis-BN-Tolan (388 nm, FFL = 0.020) is shifted to an even longer wavelength. The fluorescence quantum yields (FFL) of both BN tolan derivatives are modest, however, relative to tolan these efficiencies are improved.
Copyright © American Chemical Society