AmericanChemicalSociety.com
Reports: AC9 47421-AC9: Stability Behavior of Clay/Nanoparticle Suspensions
John Y. Walz, Virginia Polytechnic Institute and State University
Overview of Project
The goal of this project is to understand the formation mechanisms and properties of a freeze-dried composites produced from binary mixtures of silica nanoparticles and kaolinite clay particles. Upon mixing aqueous solutions of kaolinite, silica nanoparticles and salt, a gel is formed which is then freeze-dried. The composites display a porous structure arising from the formation and propagation of ice crystals during freezing (termed freeze casting). The addition of kaolinite clay particles to the dispersion used in synthesizing the composite was found to alter significantly the size and shape of the pores, as well as the mechanical strength of the composite.
Focus During Previous Year
There were two important issues that were questions during the previous year.
(1) What is the effect of freezing-rate on the structure of the freeze-dried composites, and is this effect different for the sample surface versus the interior?
(2) What is the fundamental mechanism by which the kaolinite particles increase the strength of the composites?
The results of our studies on these issues are summarized below.
Effect of freezing-rate on surface and interior structures
Samples containing different ratios of silica and kaolinite were prepared and frozen in a commercial freeze-dryer at freezing rates of either 0.05 K/min or 2 K/min. The total volume fraction of each sample (volume fraction of silica nanoparticles plus volume fraction of kaolinite platelets) was held fixed as 18%, meaning that as the kaolinite concentration was increased, for example, the silica concentration was decreased by an equal amount.
After sublimation of the water under vacuum, both the surface of the samples as well as the interior (accessed by carefully breaking the samples) were examined using scanning electron microscopy. A summary of the results from this analysis is provided in the table below.
Freeze-rate | Top Surface |
| Interior |
2 K/min | As kaolinite concentration increases, pores become very thin, more isolated, and much fewer in number. |
| As kaolinite concentration increases from 0% to 14% vol., pore size increases from ~5 mm to ~ 25 mm. Pores also appear to become more interconnected, and kaolinite particles form the walls of the pores. |
|
|
|
|
0.05 K/min | As kaolinite concentration increases, pores become more isolated, however pore size and shape do not significantly change. |
| With no kaolinite, pores are extremely small, existing mainly between the nanoparticles. As kaolinite concentration increases, pore size increases significantly. |
The primary conclusion from these results is that the freezing rate has much more of an effect on the structure of the sample surface than on the interior structure. In addition, the relative concentration of silica and kaolinite in the samples was found to have a much greater impact than freezing rate, especially on the interior structure.
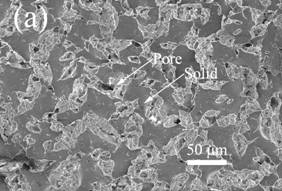
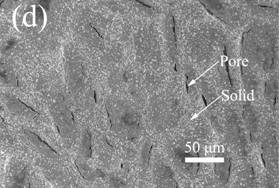
SEM micrographs showing representative samples of the surface and interior are shown below.
SEM images of the top surface of 2 K/min freeze-cast composites with
different kaolinite concentrations: (a) 0% vol. kaolinite, (d) 14% vol.
kaolinite. The scale bar is the same in all figures to facilitate comparisons. SEM images of the interior of 0.05 K/min freeze-cast composites with
different kaolinite concentrations: (a) 0% vol. kaolinite, (d) 14% vol.
kaolinite. Fundamental mechanism
responsible for increase in sample strength by kaolinite One of the most significant findings of our prior work was
that the addition of the kaolinite particles has a substantial impact on the
strength of the freeze-dried composites.
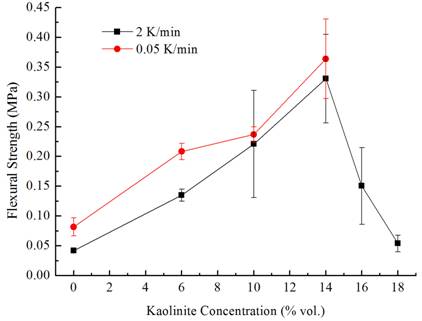
This can be seen clearly in the graph below, which shows the equibiaxial flexural strength of the composites as a
function of kaolinite concentration at the two different freeze rates. As seen, the effect of the freeze-rate is not
nearly as significant as the kaolinite/silica ratio.
Equibiaxial flexural strength of freeze-cast composites
measured at 2 K/min and 0.05 K/min freezing rates. The error bars indicate the
standard deviation of the measured values. Our hypothesis for this increased strength was that the
silica nanoparticles strongly bind to the kaolinite platelets and that the
large kaolinite plates hold the bonded network together, allowing load to be
transferred more efficiently. The
decrease in strength seen at kaolinite concentrations above 14% vol. (silica
concentrations below 4% vol.) arises because there is insufficient silica
particles present to form the connecting network.
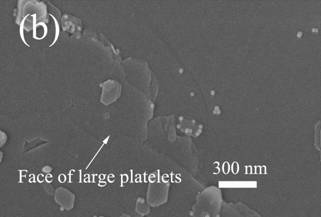
To test this hypothesis, some simple adsorption measurements
were performed in which very dilute aqueous solutions of silica and kaolinite
were mixed together. The kaolinite
particles were then allowed to settle and were recovered and washed for SEM
analysis. Shown below are SEM micrographs
of the recovered platelets. As seen,
when NaCl is present in the system, which is the case
for all samples discussed above, there is substantial adsorption on the faces
of the platelets.
SEM images of sedimented kaolinite plates
after adsorption tests. The solution used in (a) contained 0.5 M NaCl, while the solution used in (b) contained no added
salt These results
indicate clearly that the significant increases in strength of the freeze-cast
composites produced by the addition of kaolinite arises from the ability of the
kaolinite plates to bond together relatively large portions of the
nanoparticles network and increase the strength of the network structure
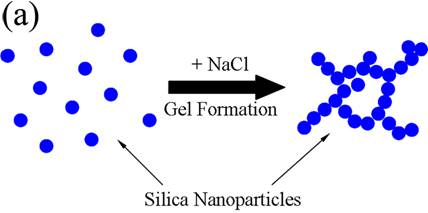
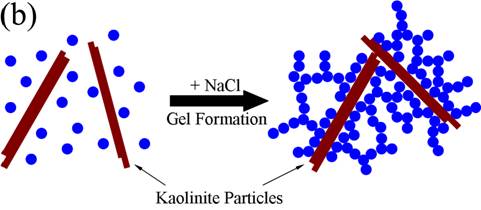
(schematic below). Upon freeze-casting
the gels, the growing ice-crystals will obviously disrupt this network and a
porous structure is formed. Nonetheless, the increased strength of the
composite produced by the kaolinite-silica particle interaction clearly still
persists. Based upon the nitrogen adsorption measurement, the specific surface
area of particles is conserved during freeze casting, indicating that the sphere-like
nanoparticles interact with each other by point contact. When an external load
is applied to the sample, the point-to-point contacts from the nanoparticles
are not able to transfer the load effectively, thus the strength of pure silica
sample is low. When kaolinite particles are present, the platelets are bonded
together by nanoparticles throughout the network
Proposed gelation
mechanism. (a) without
kaolinite, the nanoparticles aggregate
into a porous network upon the addition of salt, (b) when kaolinite plates are added, the
platelets are bound together by nanoparticles to form network, adding strength
to the structure.
Copyright © American Chemical Society