AmericanChemicalSociety.com
Reports: B3 48311-B3: Synthesis, Structural Determination, and Physicochemical Property Studies of Novel Coordination Polymers Incorporating Kinked and Hydrogen-Bonding Capable Bifunctional Organodiimines
Robert L. LaDuca, Michigan State University
Over the past year the undergraduate-only LaDuca research group has explored the synthesis, structural characterization, and physical property investigation of divalent metal dicarboxylate coordination polymers containing hydrogen-bonding capable and flexible tethering dipyridyl ligands. During the timeframe September 2009 to August 2010, the group published 18 articles (13 papers/communications, 5 structural reports), and has 5 articles in press. Two of the papers were invited submissions for journal special issues in Polyhedron and CrystEngComm, highlighting the work of talented young investigators. Stipends for two undergraduate researchers were covered by the grant. Both students are women, with one being a member of an underrepresented ethnic minority.
The undergraduates were deeply and independently involved in every aspect of the work: organic synthesis of the dipyridyl ligands, low- and high-temperature inorganic synthesis, single crystal X-ray crystallography, molecular graphics rendering, variable temperature magnetic susceptibility studies, emission spectroscopy, and thermogravimetric analysis. They also assisted in manuscript preparation. It is clearly evident that they developed laboratory and analysis techniques during their time in the research laboratory. Perhaps more importantly, they developed these capabilities while preparing and analyzing new coordination polymer compounds, not while performing well-worn procedures.
The PI does not host any graduate students or postdoctoral fellows, because of his unique appointment within Lyman Briggs College at Michigan State University (an under-graduate-only residential college for the study of science and its impact on society). Thus the advancement of his research program depends critically on the hard work of these undergraduates.
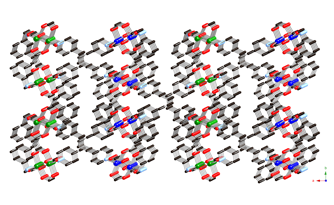
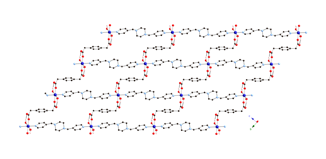
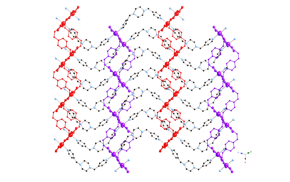
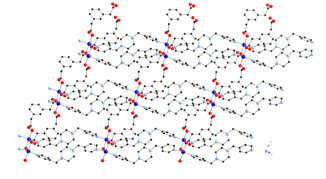
Much of the research carried out in the 2009-2010 reporting period involved the synthesis, structural characterization, and physical property measurements of divalent metal coordination polymers incorporating the seldom-used phenylenediacetate (phda) and phenylenedipropionate (phdp) ligands, along with dipyridyl nitrogen co-ligands.
Divalent copper
coordination polymers containing flexible dipyridylpropane
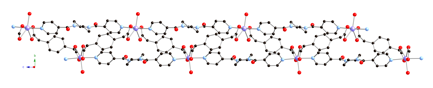
(dpp) and phenylenediacetate
(phda) ligands display different metal cluster
subunits and self-penetrated three-dimensional (3-D) topologies, depending on
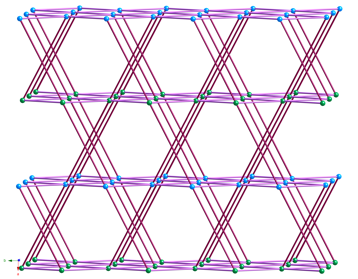
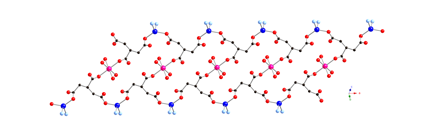
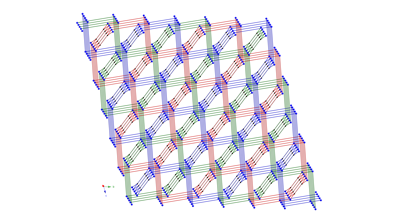
the disposition of the pendant arms within the anionic components. {[Cu2(1,3-phda)2(dpp)]n (1) possesses dinuclear{Cu2(OCO)4}
paddlewheel subunits linked into a 6-connected self-penetrated rob lattice with 48668
topology. {[Cu4(1,4-phda)3(OH)2(dpp)2]¥2H2O}n (2)
manifests non-cubane tetrameric
{Cu4(OH)2}6+ kernels, connected into an
8-connected self-penetrated ilc lattice with 4245.63 topology. Antiferromagnetic coupling within the
metal cluster subunits is observed in both 1
and 2.
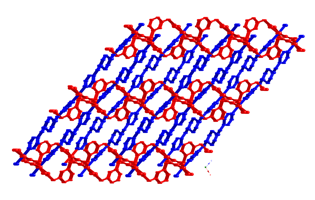
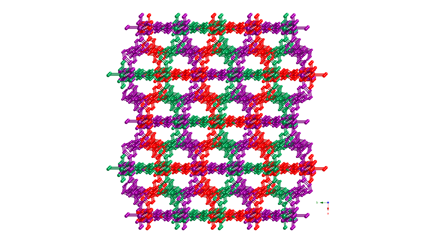
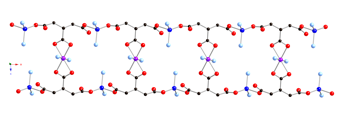
1 Divalent cobalt
coordination polymers containing both phenylenediacetate
(phda) and bis(4-pyridylmethyl)piperazine (4-bpmp) ligands display
differing dimensionalities and topologies. The para-substituted 1,4-phda ligand
afforded {[Co(1,4-phda)(4-bpmp)(H2O)2]¥2H2O}n (3), a layered phase with standard (4,4) rhomboid grid
topology. Use of the meta-substituted 1,3-phda ligand
generated [Co(1,3-phda)(4-bpmp)]n (4), which
has [Co(1,3-phda)]n layers
pillared by pairs of 4-bpmp ligands into a three-dimensional (3-D) coordination
polymer with primitive cubic topology. Antiferromagnetically
coupled {Co2(CO2)2} dinuclear units (J
= –2.04(4) cm–1)
are embedded within the structure of 4. The ortho-substituted
derivative {[Co(1,2-phda)(4-bpmp)1.5(H2O)](H24-bpmp)0.5(ClO4)¥12H2O}n (5) manifests an intriguing layered structure with 5-connected
cobalt atom nodes and an Archimedean 334453 cem topology
with triangular and rectangular circuits.
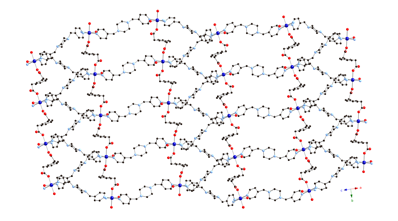
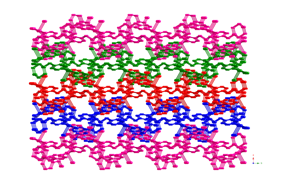
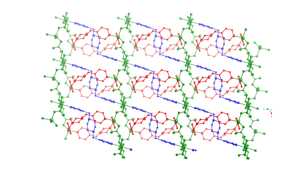
3 4 5 Hydrothermal synthesis has also afforded
divalent cadmium coordination polymers containing bis(pyridylmethyl)piperazine
(bpmp) tethers and either phenylenediacetate
(phda) or phenylenedipropionate
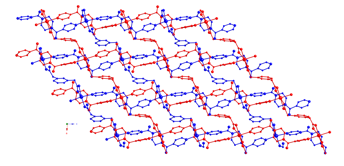
(phdp) ligands. {[Cd(1,4-phda)(4-bpmp)]¥1.5H2O}n (6) displays a (4,4)-grid layered structure based on 4-connected {Cd2O2}
dimeric units.
Extension of the pendant arms generated {[Cd(1,4-phdp)(4-Hbpmp)](ClO4)¥3.5H2O}n (7, phdp = phenylenedipropionate),
which possesses a rare (3,6) 2-D trigonal lattice
based on 6-connected {Cd2O2} dimers. Changing the nitrogen donor atom
disposition by using 3-bpmp as the nitrogen co-ligand yielded [Cd(1,4-phdp)(3-bpmp)]n (8), which crystallizes in a 3-fold interpenetrated achiral diamondoid lattice.
Complexes 6–8 undergo blue-violet luminescence upon
exposure to ultraviolet radiation.
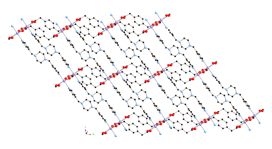
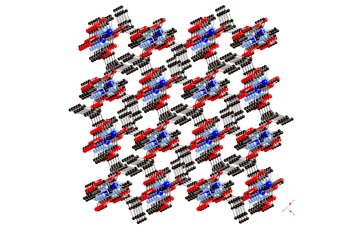
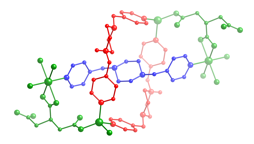
6 7 8 Three divalent
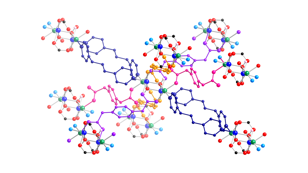
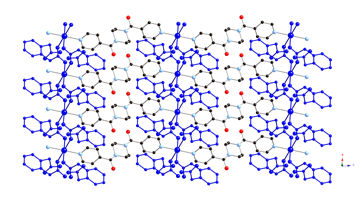
copper coordination polymers containing aromatic dicarboxylate
ligands and the long-spanning tethering imine bis(4-pyridylmethyl)piperazine
(4-bpmp) have been prepared and structurally characterized. The length of the dicarboxylate pendant arms, carboxylate binding mode, and
the inclusion of anionic components play a synergistic role in structure
direction in this system. {[Cu(ip)(4-bpmp)(H2O)]¥5H2O}n
(ip = isophthalate, 9) displays neutral (4,4) rectangular
coordination polymer grids that stack in an ABCD
repeat pattern. Use of the longer
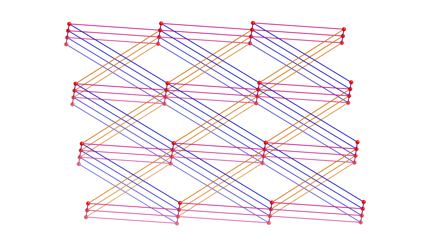
pendant arm dicarboxylate 1,3-phenylenediacetate (phda) resulted in {[Cu2(phda)2(4-bpmp)]¥H2O}n (10), a 3-D network coordination polymer with primitive cubic
topology that features strongly antiferromagnetically
coupled (J = –331(1) cm–1)
{Cu2(CO2)4} paddlewheel units. In the presence of excess nitrate ions,
{[Cu(phda)(H4-bpmp)](NO3)¥3H2O}n (11) was isolated instead of 10;
11 manifests cationic 2-D
coordination polymer layers built from weakly antiferromagnetically
coupled (J = –2.43(1) cm–1)
{Cu2O2} dimers linked through phda
and protonated bpmp
ligands. The striking difference
in magnetic properties is ascribed to the equatorial-equatorial
versus axial-equatorial bridging of
copper coordination spheres in 10 and
11, respectively.
9 10 11 We have also begun to investigate the
coordination polymer chemistry of flexible, aliphatic tricarboxylate
ligands. Hydrothermal reaction of zinc perchlorate, tricarballylic acid (H3tca) and bis(4-pyridylmethyl)piperazine
(bpmp) resulted in generation of {[Zn3(tca)2(bpmp)(Hbpmp)2](ClO4)2 ¥5H2O}n (12), which possesses an unprecedented self-penetrated
two-dimensional (2-D) layered topology with threaded-loop type linkages. A similar reaction using zinc sulfate
as precursor afforded the oxoanion-free phase {[Zn3(H2O)4(tca)2(bpmp)2]¥8H2O}n (13), which manifests a new yet simple three-fold interpenetrated (63)(658)
3,4-connected 3-D binodal network.
Both materials undergo blue-violet luminescence upon exposure to UV
radiation.
12 13 More recently, our group's efforts have
focused on utilizing bis(4-pyridyl-formyl)piperazine (bpfp)
as a neutral tethering ligand in coordination polymer synthesis. This ligand
has hydrogen-bonding acceptance ability at its formyl
groups, and avoids piperazinyl-based hydrogen-bonding
donation through amide resonance forms.
In some preliminary cases, this adjustment in hydrogen-bonding facility
results in different coordination polymer topologies than those seen in related
4-bpmp phases. Hydrothermal synthesis has generated
divalent cadmium coordination polymers containing phenylenediacetate
(phda) and bis(4-pyridylformyl)piperazine (bpfp)
ligands, in which the position of the pendant acetate arms plays a very
significant role in structure direction during self-assembly. [Cd(1,2-Hphda)2(4-bpfp)]n (14)
exhibits 2-D polymeric layers with embedded anti-syn bridged [Cd(OCO)2]n
ribbons. {[Cd(1,3-phda)(4-bpfp)(H2O)]2}n (15) has crystallographically independent (4,4) grids with different
carboxylate binding modes, engaged in parallel interpenetration. {[Cd(1,4-phda)(4-bpfp)(H2O)]}n (16) manifests acentric 1-D chains with an
uncommon 4-connected 33425 topology instilled by the
mismatch in ligand length. Luminescent properties of all phases are also
reported.
14 15 16 Work continues in our laboratory in this
fertile research vein. While the
project has borne significant scientific fruit, the intellectual development of
the undergraduates involved in the research remains first and foremost.
Copyright © American Chemical Society