AmericanChemicalSociety.com
Reports: UR10 49390-UR10: Enhancing Conversion Efficiency of Dye-Sensitized Solar Cells by Synthesis of Highly Ordered Titania Structures and Judicious Selection of Redox Couples
Feimeng Zhou, California State University (Los Angeles)
Summary:
We have (1) synthesized negatively charged CdS quantum dots (QDs), (2) immobilized the QDs onto ITO (indium-tin oxide) substrate and fabricated the CdS QDs semiconductive photoelectrodes by the layer-by-layer assembly technique, (3) fabricated photoelectrodes covered with multilayered QDs and different sensitizers or dye molecules, (4) set up the apparatus for photoelectrochemical measurements and (5) investigated the photoelectrochemical behaviors of these electrodes. UV absorption and fluorescence measurements confirmed that stable, uniform CdS QDs had been synthesized. The negatively charged CdS QDs can be readily immobilized onto conductive ITO substrate by alternately assembling polydiallyldimethylammonium and QDs. Such an approach allows precise control of the thickness of the QDs film. In order to study the effect of different sensitizers on the photoelectrochemical behavior of the as-prepared electrodes, we have tested a few different sensitizers of for their spectral and photochemical properties (See Figure 1 for the sensitizer structures).
Figure 1. Structures of Pt-containing photosensitizers. The photoelectrochemical set-up is shown in
Figure 2a. Figure 2b shows the photocurrent
response at an electrode covered with multilayered QDs. A significant
photocurrent was observed, indicating the QD-modified electrode is capable of
converting light energy into electrical current. A closer analysis of the
current polarity upon irradiation reveals that a photooxidation
process takes place. We found that electrodes constructed with QDs that were synthesized
with different CdS and citrate ratios produced
different amounts of photocurrent. The influence of the number of layers on the
photoelectrochemical performance was also
investigated. Electrode assembled for ten layers shows the best photoelectrochemical performance.
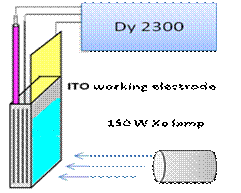
b Figure 2. The home-made fluorescent spectrophotometer (a) and the photovoltaic current
response at the (PDDA/QDs)5 film (b). DY
2300 is a bipotentiostat. We also investigated the sensitizing effect of the sensitizer molecules
shown in Figure 1. Interestingly, the sensitizer-coated multilayer QDs photoelectrode behaves differently from the sensitizer-free
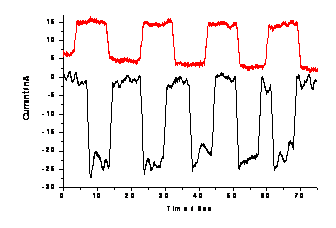
electrode. Figure 3 shows photoelectric current response at a PDDA/QD film coated
with FcNNNPtCl. Upon irradiation, instead of
oxidation current, a reduction current was observed. The other
two sensitizers (CH3phNNNPtCl and (CH3)2NphNNNPtCl),
despite their structural and photochemical differences, exhibit similar photoelectrochemical behavior to the FcNNNPtCl.
The finding in the current switch may be of both fundamental and applied
significance. Further investigation into the mechanism and possible application
is underway. Figure 3. Photovoltaic current responses at a PDDA/QD film (black
line) and a PDDA/QD film coated with FcNNNPtCl (red
line). Impacts of Research on the PI and his
Student: This grant enabled the PI to apply his expertise (electrochemistry,
surface chemistry and material synthesis) to energy-related research. Performing
such research allowed the PI to interact with other researchers in an
NSF-funded center (Center for Energy and Sustainability) and to initiate
related research activities (e.g., fuel cell testing and development and
catalysis synthesis and characterization). Angela Ramos, an undergraduate
chemistry major partially supported by an NSF-REU (Research Experience for
Undergraduates) had worked closely with the PI on this project in the summer of
2010 and in the academic year between 2009 and 2010 through directed
undergraduate research. As the research activities have drastically expanded in
the past year, she will be supported by this grant starting from September 2010
so that more effort can be made to ensure the progress. Conducting research in
the PI's group has exposed Angela to various research fields and motivated her
to pursue an advanced degree in chemistry.

Copyright © American Chemical Society