AmericanChemicalSociety.com
Reports: G9 47954-G9: Latex Composite Ultrafiltration Membranes
Subramanian Ramakrishnan, Florida State University
Latex Composite Ultrafiltration Membranes: Report
Subramanian Ramakrishnan (PI)
ACS PRF# 47954-G9
This proposal seeks to overcome current difficulties in current membrane manufacture by utilizing the advantages of particle self-assembly combined with tailoring particle surfaces to fabricate membranes with a wide range of narrow pore size distributions and surface compositions. The focus will be to synthesize ultrafiltration (UF) membranes (10 – 50 nm in pore sizes) with higher fluxes and narrower pore size distributions than commercial membranes. By placing an array of colloidal particles on a porous support and stabilizing the array, the interstitial spaces between the particles serve as narrow dispersed pores for size separations.
Results (since August 2009)
1) Synthesis and Characterization of Latex Composite Membranes: Monodisperse Particles
Particles of 200 nm, 650 nm and 900 nm were successfully synthesized using an emulsion polymerization technique. Membranes were then fabricated by depositing these particles on commercial supports and by heat stabilizing them.
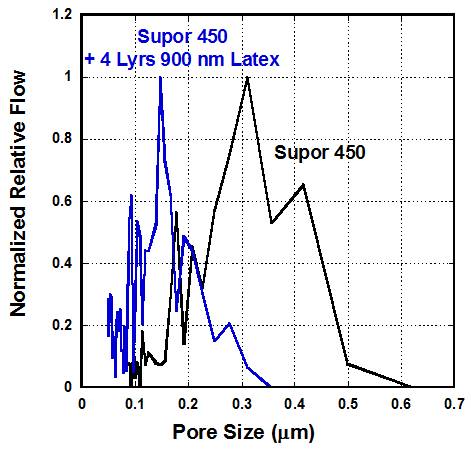
Figure 1: Pore size distribution of Supor 450 and 4
layers of 900nm latex deposited on top of Supor 450.The peak in pore size
decreases to lower pore sizes with addition of particles and also results in a
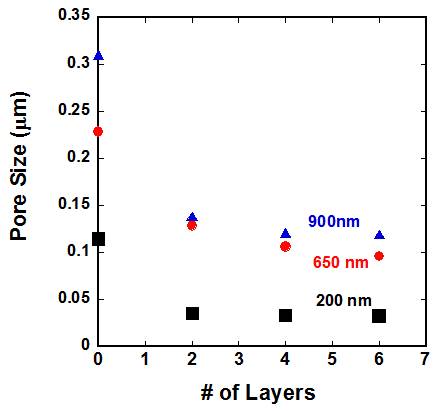
narrower pore size distribution. Figure 2: Pore size as
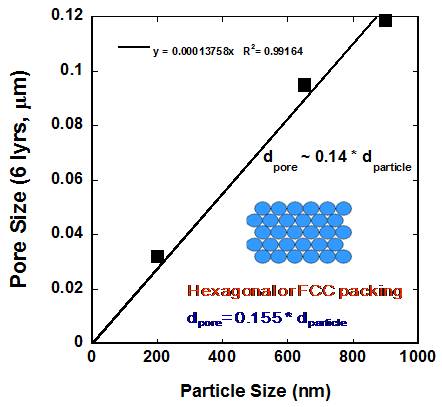
a function of number of layers for different size particles used in this work. Figure 3: Pore size for 6 layers of particles as a
function of particle size. It is found
that the pore diameter is approximately 14 % of the particle size which is
slightly lower than a hexagonal or FCC packing. Figure 4 Comparison of
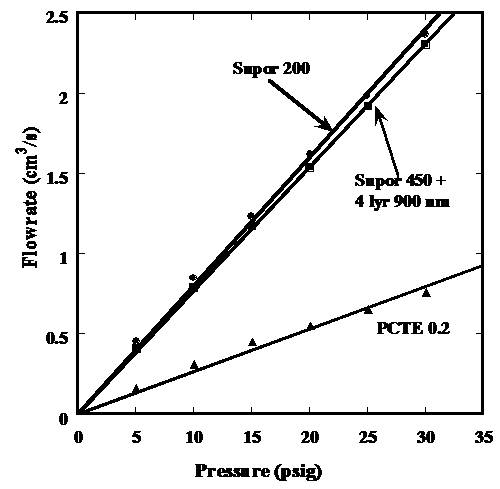
water flow rate data for Supor 200, Supor 450 with approximately 4 layers of
deposited 900 nm polystyrene particles, and PCTE 0.2 Figure 4 demonstrates that when 4 layers of 900 nm
particles are deposited on a base membrane of Supor 450, a water flux
comparable to that of Supor 200 is achieved.
The advantage here is that the latex composite membrane has a pore size
of 0.12 microns while the Supor 200 has a pore size of 0.23 microns. Thus using this technology one has formed a
membrane with a narrower pore size and higher flux. 2)
Synthesis and Characterization
of Latex Composite Membranes: Particle
Mixtures
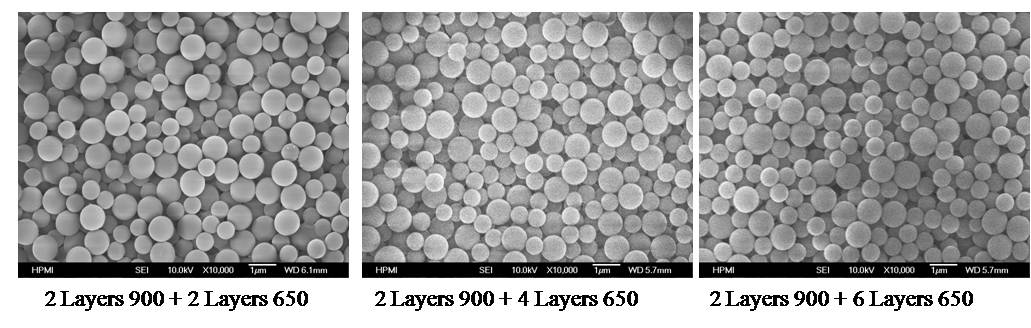
Figure 5: Top View SEM
Images of Multi Size Mixed Packed Beds on Supor 450 Figure
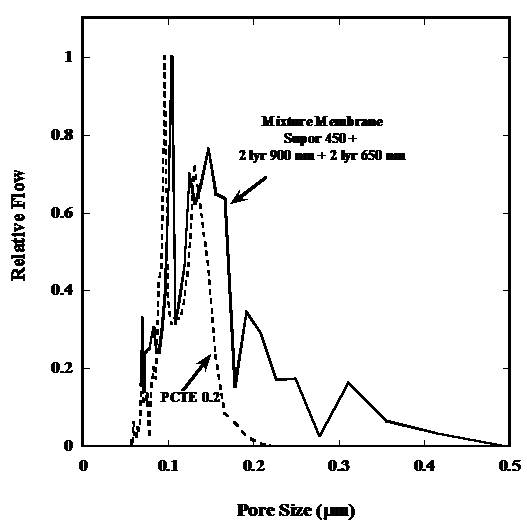
6: Comparison of normalized relative
flow as a function of pore size for PCTE 0.2 and mixture membrane made up of 2
layers 900 nm and 2 layers of 650 nm particles on Supor 450
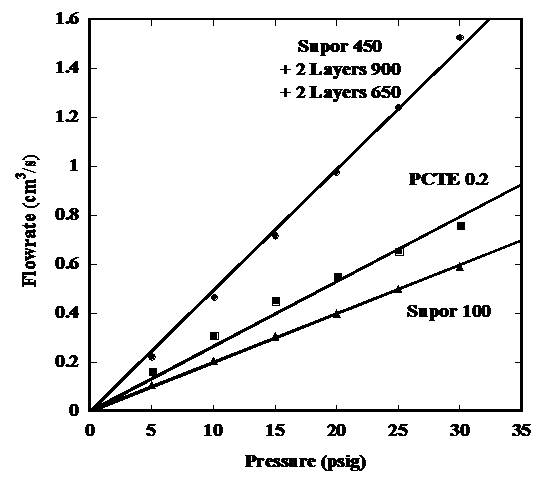
Figure 7. Comparison of flow rate as a function of applied pressure
for Supor 100, PCTE 0.2, and mixture membrane made up of 2 layers 900 nm and 2
layers of 650 nm particles on Supor 450 Overall Conclusions: It is possible to have good control of pore
sizes and their distribution by forming membranes from colloidal particles. The pore sizes were ~ 14% of the particle
diameter and the water fluxes could be predicted using the Carmen-Kozeny
equation for packed beds. Using mixtures
of particles, it was shown that one could achieve smaller pore sizes (~ 28 nm)
and that one could achieve pore size distributions similar to commercial track
etched membranes but at the same time have higher fluxes. A paper
based on current work is under preparation for submission to a peer reviewed
journal. Impact of Research 1) This
work resulted in the first Masters Student (Erin
Holley) graduate through the Materials Science program at Florida State
University. 2) It
is playing a key role in the undergraduate education of Velencia Witherspoon
and Djeri Harris (both African American
women) who are working in my lab.
Both of them want to pursue graduate school after their undergraduate
studies.
Copyright © American Chemical Society