AmericanChemicalSociety.com
Reports: DNI7 49220-DNI7: Templating Synthesis of Polymeric Nanocages via Interfacial Polymerization of Monomer-Functionalized Surfactant Monolayer Absorbed on Crystallized Microemulsion Nanodroplets
Chong Cheng, PhD, State University of New York at Buffalo
The objective of this research project is to establish a new and facile method for the preparation of well-defined polymeric nanocages by achieving one-to-one polymerized copies of surfactant monolayers adsorbed on crystallized microemulsion nanodroplets. With charged amphiphilic monolayer-thick shell, the targeted polymeric nanocages potentially may have broad applications in controlled delivery, phase transfer catalysis, nanoreactor, and layer-by-layer preparation of complex nanomaterials. Two synthetic approaches were designed and currently are under investigation. In the first approach, monomer-functionalized surfactants need to be synthesized at first, and then converted into nanocages by interfacial crosslinking via crystallized microemulsion nanodroplets. In the second approach, crystallized microemulsion nanodroplets are prepared using common surfactants, and used for the synthesis of nanocages by surface-absorption of charged monomers, followed by interfacial crosslinking.
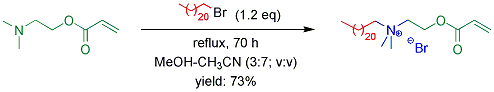
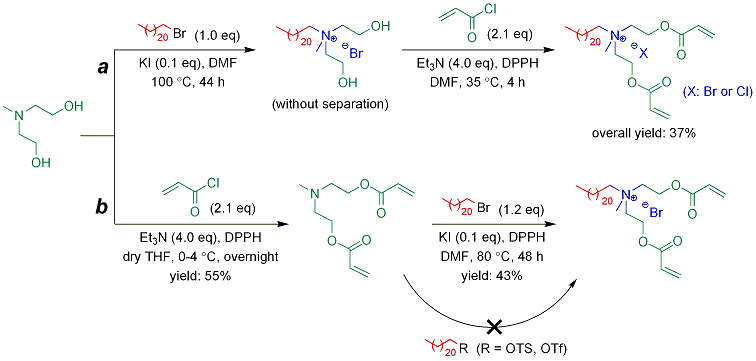
Significant research progress has been made in the investigation of the first approach. Quaternary ammonium-based cationic monomer surfactant (with one acryoyl group) and crosslinker surfactant (with two acryoyl groups) were synthesized. Monomer surfactant was obtained in 73% yield by the quaternization reaction of 2-(dimethylamino)ethyl acrylate with 1-bromodocosane (1.2 eq) in 30 vol% methanol-acetonitrile under reflux for 70 h (Scheme 1). The preparation of crosslinker surfactant was more challenging (Scheme 2). In a one-pot, two-step process, crosslinker surfactant was synthesized with 37% overall yield. In the first step, quaternization reaction of N,N-bis(2-hydroxyethyl)methylamine with 1-bromodocosane (1.0 eq) was conducted using potassium iodide (0.1 eq) as catalyst, in DMF at 100 ºC for 44 h. The resulting quaternized product was insoluble in common organic solvents at room temperature, and therefore separation using flash chromatography could not be performed. Thus, esterification reaction was directly conducted by adding acryloyl chloride (2.1 eq), triethylamine (4.0 eq), and a trace amount of 2,2-diphenyl-1-picrylhydrazyl (DPPH; a polymerization inhibitor) into the reaction mixture at 35 ºC, with reaction time of 4 h. The resulting crosslinker surfactant, with both bromine and chlorine anions, was obtained after purification by flash chromatography. Alternatively, crosslinker surfactant was also synthesized by the preparation of amine-based bifunctional monomer, followed by quaternization reaction. But this route resulted in relatively low overall yield (not more than 24%). The esterification reaction of N,N-bis(2-hydroxyethyl)methylamine with acryloyl chloride (2.1 eq) was performed in the presence of triethylamine (4.0 eq) and DPPH, in dry THF at 0-4 ºC. After overnight reaction, the resulting monomer was obtained in 55% yield after column separation. The yield of quaternization was 43% for the reaction of the monomer with bromodocosane (1.2 eq) in the presence of potassium iodide (0.1 eq) and DPPH, in DMF at 80 ºC for 48 h. If using 30 vol% methanol-acetonitrile as solvent under refluxing (without potassium iodide), the reaction yield was only 21% after 7 days. In order to improve the yield of quaternization, instead of bromodocosane, p-toluenesulfonic acid n-docosanyl ester (n-C22H45-OTs) and trifluoromethanesulfonic acid n-docosanyl ester (n-C22H45-OTf) were prepared and used to react with the amine-based monomer. However, although various reaction conditions were attempted, the sequential quaternization reaction failed (0-5% yield), presumably due to the great steric hindrance for the reaction systems.
Scheme 1. Synthesis of monomer surfactant
Scheme 2. Synthesis of crosslinker surfactant
The preparation of crystallized nanodroplets using monomer-functionalized surfactants via oil-in-water microemulsion has been investigated. With choosing docosane (melting point: 43-45 ºC) as oil, microemulsions were prepared at 55 ºC after ultrasonication, and then the system was cooled down to room temperature to obtain crystallized nanodroplets. Dynamic light scattering (DLS) was used to monitor whether the resulting crystallized nanodroplets have significant stability allowing for sequential templating synthesis. Only monomer surfactant was used in the preliminary study, and well-defined nanoscopic crystallized nanodroplets were obtained in some trials. For instance, a microemulsion system (wt. of water: wt. of monomer surfactant: wt. of docosane = 90: 6: 4) resulted in transparent dispersion of crystallized nanodroplets with sizes of 4-5 nm. However, for many trails, phase separation was observed hours after crystallized nanodroplets were obtained. With a DLS instrument installed in the PI's laboratory this August, the research work on nanodroplet crystallization control has been accelerated. As soon as the optimal conditions for the preparation of well-defined, relatively stable crystallized nanodroplets are established, the investigation of nanocage synthesis by crosslinking the monomer groups at water-oil interface will be conducted.
Given the challenges in the synthesis and purification of monomer-functionalized surfactants, the approach for the preparation of nanocages via surface-adsorption of charged monomers is attractive because common surfactant can be used to prepare crystallized microemulsion nanodroplets. The corresponding investigation currently is in the initial stage. Crystallization process for the microemulsions prepared from water, docosane, and sodium dodecyl sulfate (SDS) has been studied. Well-defined crystallized nanodroplets have been obtained in some trials. In next steps, the crystallized nanodroplets will be treated with quaternized monomer and crosslinker, and then interfacial crosslinking of the adsorbed surface layers of monomer and crosslinker will be investigated for the preparation of nanocages.
Copyright © American Chemical Society