AmericanChemicalSociety.com
Reports: G7 47926-G7: Graphene Nanoribbons: Synthesis and Self-Assembly of Nanostructured Materials
C. Scott Hartley, Miami University
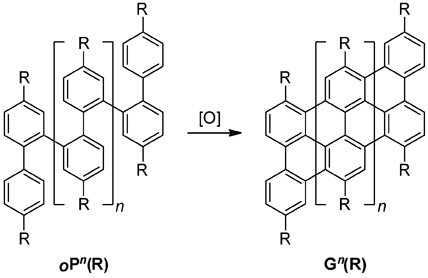
Over the final year of our Type G grant, we have made significant progress in our project to understand, characterize, and derive new materials from ortho-phenylenes. Briefly, the goal of this project was the development of synthetic methods toward ortho-phenylene oligomers (oPn), and their subsequent oxidative planarization to graphenes Gn. Graphene, a two-dimensional sheet of benzenoid carbon atoms, has recently attracted great attention due to its unusual and highly efficient charge-transport behavior. Compounds Gn represent the extreme limit of miniaturization of graphene-based structures, for use as fundamental models for comparison with theoretical approaches to the electronic structure of graphene, as candidate molecular wires, and as self-assembled materials (e.g., liquid crystals).
Our principal effort over 2009–2010 was to apply the conditions we had already developed for the synthesis of oPn to create significant quantities of a homologous series of oligomers. Accordingly, we were able to prepare >300 mg of each of oP4, oP6, oP8, oP10, and oP12 (R = OMe). With access to these materials, we have found that delocalization (as measured by UV/vis spectroscopy) extends remarkably far along the o-phenylene backbone. The compounds exhibit other unusual properties compared to most conjugated oligomers or polymers, particularly hypsochromic shifts in fluorescence spectra with increasing length, and essentially invariant first oxidation potentials. We speculated that these properties likely result from the conformational behavior of the ortho-phenylenes, and are consistent with a well-defined stacked helical conformation. A paper describing these results has recently been published in J. Am. Chem. Soc.
We then continued this study by using variable temperature NMR and computational chemistry to analyze the conformational behavior of the o-phenylenes. Briefly, we have found that in all cases the overall conformational pool is dominated by a single twofold-symmetric conformation, and we have established that this conformation does indeed correspond to a stacked helix. Significantly, our results suggest that the o-phenylene motif may be a powerful means to organize chromophores in three dimensions. This could lead to new model systems for the study of through-space energy and charge transfer. A manuscript on the conformational behavior of the ortho-phenylenes has been submitted for publication.
Our study of the direct planarization of oPn to Gn continues, although it has not yet been successful. As discussed in the previous year's report, the direct oxidative cyclodehydrogenation using a combination of Lewis acid and oxidant (the Scholl reaction) has generally proven unsuccessful, consistent with the results of King and co-workers (Tetrahedron 2008, 64, 11370). Although the focus of the year's work has been the studies described above, we continue to test new conditions as they are developed and we are currently exploring other possible precursors to the desired graphenes.
The funding from our PRF-G grant has had a substantial impact on this project, and on my career and those of my students. Synthetic organic chemistry is, of course, a costly endeavor, and this grant was used directly to purchase needed reagents and supplies early in the project. Perhaps most significantly, the grant allowed us to purchase several significant pieces of equipment that were heavily used in our first publication on the o-phenylenes (UV/vis and fluorescence spectrometers). Hands-on access to this equipment allowed us to tease out some of the more subtle properties of these molecules, and it is fair to say that we would have been unable to carry out some key experiments for that paper without long term access to the instruments.
Over the grant period, the funding from the PRF directly supported supplies for 1 postdoctoral fellow, 1 graduate student, several undergraduates, and myself. One of the undergraduates was also supported by a Supplement for Underrepresented Minorities, which was discussed in its own annual report. We have presented our results at several regional and national conferences, and two papers based directly on our PRF-funded work are published or submitted. Further, the results we obtained during the grant period were used as the basis for a successful grant application to the National Science Foundation. This new funding will sustain the project over the coming years.
Copyright © American Chemical Society