AmericanChemicalSociety.com
Reports: B3 46064-B3: Vanadium-Scorpionate Coordination Complexes: Syntheses, Characterization, and Reactivities
Craig C. McLauchlan, PhD, Illinois State University
Vanadium coordination chemistry lies at the heart of the research interests of the PI and his research group. This proposed research outlines synthetic methods and characterization techniques to study new vanadium-organophosponates with relatively porous molecular structures prepared from vanadium(III) and (IV) precursors.
The goal of the proposed work is to develop synthetic methodologies in the preparation of vanadium- organophosphonate complexes and to completely characterize these complexes. A series of tridentate scorpionate ligands with O and/or N binding moieties are being employed to direct the stereochemistry involved and direct the formation of lower dimensional structures. The long term goal is, ultimately, to study these complexes for catalytic activity. The project can be roughly divided in to four main areas:
1) Synthesis of molecular cages of V(III) complexes with organophosphonates
2) Synthesis of a parallel series of V(IV) complexes
3) Characterization of synthesized products.
4) Studies on the catalytic activity of the synthesized products.
Our initial studies have focused on goals 1-3. Here we report the progress on each area.
1) Synthesis of molecular cages of V(III) complexes with organophosphonates
After a hiatus from this portion of the project the previous year, we returned to it during this past 12 months. We still focused the bulk of our resources and time pursuing goal #2, where more significant headway was being achieved, but this area is still a desired goal of the project. Two undergraduate researchers worked on this during the school year and were successful in repeating the work our lab had already shown, but, alas, no further progress was made. It is expected that these now-trained students will make progress on this area in the coming months.
2) Synthesis of a parallel series of V(IV) complexes
 During the
past year
During the
past year
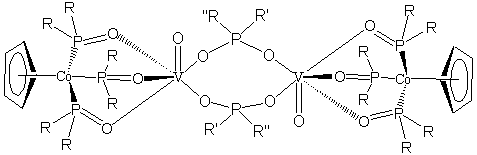
![]() we have continued
to almost exclusively make vanadium complexes with the
cyclopentadienyltris(dialkylphosphito)cobaltate(III) ligand system, CpPCo
(shown), often referred to as the Kläui ligand
(R=Me,Et for us). One of the advantages
of the CpPCo ligand to this project is the sterically directing nature of the
ligand it is tripodal/tridentate as with the scorpionate ligands, but with
oxygen as the binding atom. The O,O,O-bonding nature of this ligand
stabilizes the oxophilic vanadium that may allow us to make molecular
structures. Previously, we have reported
a series of V(III) and V(IV) complexes with the CpPCo ligand with a number of
co-ligands. These complexes have proven
surprisingly robust and the substitution chemistry of the CpPCoVCl2(DMF)
allows facile preparation of the series with co-ligands such as oxalate or
another equivalent of CpPCo. Of late we
have been using the oxidized material, nominally CpPCoVOCl(DMF), with similar
substitution chemistry to generate a series of bridged phosphate, phosphinate,
and phosphonate dimers. A generalized
structure is shown below. The
stereochemistry of the vanadyl complexes makes formation of the dimers much
more easily accomplished than tetramer formation and we have exploited that
advantage. Dimers have proven to be a
better structural model of our catalyst and we hope they will prove to be a
better functional model as well.
we have continued
to almost exclusively make vanadium complexes with the
cyclopentadienyltris(dialkylphosphito)cobaltate(III) ligand system, CpPCo
(shown), often referred to as the Kläui ligand
(R=Me,Et for us). One of the advantages
of the CpPCo ligand to this project is the sterically directing nature of the
ligand it is tripodal/tridentate as with the scorpionate ligands, but with
oxygen as the binding atom. The O,O,O-bonding nature of this ligand
stabilizes the oxophilic vanadium that may allow us to make molecular
structures. Previously, we have reported
a series of V(III) and V(IV) complexes with the CpPCo ligand with a number of
co-ligands. These complexes have proven
surprisingly robust and the substitution chemistry of the CpPCoVCl2(DMF)
allows facile preparation of the series with co-ligands such as oxalate or
another equivalent of CpPCo. Of late we
have been using the oxidized material, nominally CpPCoVOCl(DMF), with similar
substitution chemistry to generate a series of bridged phosphate, phosphinate,
and phosphonate dimers. A generalized
structure is shown below. The
stereochemistry of the vanadyl complexes makes formation of the dimers much
more easily accomplished than tetramer formation and we have exploited that
advantage. Dimers have proven to be a
better structural model of our catalyst and we hope they will prove to be a
better functional model as well.
3) Characterization of synthesized products
To help train students, we have been characterizing our products using many techniques, including spectroscopy (NMR (1H, 13C,31P, 51V), EPR, IR, UV-vis), magnetic susceptibility, and cyclic voltammetry. MALDI mass spectrometry has already proven useful in identifying our reaction products and will help in identifying future products. The X-ray crystal structures of those compounds that have formed single crystals have also been very interesting we now have solved the structures of a series of five of the dimers. We have recently been collaborating with a local college to collect the SQUID spectra for our complexes. These measurements have shown the susceptibility of the dimers to be quite complex and we are examining the structure/magnetism correlations with the hopes of publishing a joint manuscript in the near future.
4) Studies on the catalytic activity of the synthesized products
The ultimate goal is to create well-characterized relatively open molecular structures that could be studied for oxidative catalytic activity via 1H and 13C NMR spectroscopy and by gas chromatography-mass spectrometry. Previously we had reported studies done on our simpler species using the catalyzed oxidation of 3,5-di-tert-butyl-1,2-dihdroxybenzene to the ortho-quinone species, because it is easily monitored via GC-MS. Because catalytic activity relies on easy conversion between oxidation states, it is not unexpected that, thus far, we have noted better efficiency with our V(IV) complexes as the V(IV)/V(V) couple might more easily facilitate good catalytic activity. Although not in the scope of the original proposal, we had begun a preliminary set of studies (using GC) on our series of dimers (see goal 2) to examine their efficacy as oxidation catalysts on this simple catcehol/quinone system. That work was suspended in the past 12 months and a student is being recruited to pick up this work.
Students involved in this project have presented our work internally at ISU poster sessions such as the Undergraduate Research Symposium, Graduate Research Symposium, and an end-of-summer poster session. This work has also been presented by a student at a national ACS meeting. This work has been the subject of an M.S. thesis that is about to be defended. That thesis will be turned in to a more in-depth manuscript that details the work reported here.
Copyright © American Chemical Society