AmericanChemicalSociety.com
Reports: AC7 48228-AC7: Solid Rheology of Glassy Substances: Polymer and Colloidal Systems
Gregory B. McKenna, Texas Tech University
INTRODUCTION
Colloidal systems near to the glass concentration are often taken as excellent models of the molecular glass transition temperature. Yet, one of the most important aspects of the dynamics of molecular glasses, i.e., that of structural recovery in the temperature histories catalogued by Kovacs [1], has yet to be elucidated in colloidal systems. In the present work, we take advantage of a thermo-sensitive PNIPAAM [2,3] colloidal suspension to study the aging behavior near to the glass concentration. We use multi-speckle Diffusive Wave Spectroscopy (DWS) [4] to follow the dynamics during aging. Because, unlike molecular glasses, the thermodynamic variable for colloidal systems is volume fraction and not temperature, we take advantage of the thermosensitive nature of the particles to use temperature to control the concentration. The three classical aging signatures observed in molecular glasses: Intrinsic isotherms, asymmetry of approach, and memory effect, are investigated with this colloidal suspension and the results are compared with those typical of molecular and polymeric glasses.
EXPERIMENTAL
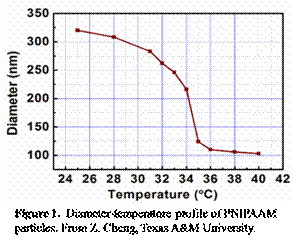
The samples we used are aqueous suspensions of PNIPAAM gel particles, which exhibit a temperature-induced swell-shrink response [2, 3]. As temperature increases from 24oC to 36oC, the diameter of the particles decreases from 350nm to 150nm (Figure 1), which means, by controlling the temperature, the system can be adjusted to different concentrations and therefore different glassy states.
 DWS
was applied to study the dynamics of the PNIPAAM colloidal system. In the
experiment, laser light is projected onto the sample and back scattered light
is collected by a CCD camera. The intensity auto-correlation
function is obtained by multiplying and averaging over pixels between two successive
frames according to [3]:
DWS
was applied to study the dynamics of the PNIPAAM colloidal system. In the
experiment, laser light is projected onto the sample and back scattered light
is collected by a CCD camera. The intensity auto-correlation
function is obtained by multiplying and averaging over pixels between two successive
frames according to [3]: ![]() . A program written in
Labview extracts relaxation time from the intensity auto correlation function.
. A program written in
Labview extracts relaxation time from the intensity auto correlation function.
The parameter used to study the
aging signature in molecular and polymer glassy systems is specific volume or
density, which are controlled by temperature (or pressure) and a volume
departure from equilibrium ![]() is used to define the
glassy state. However, in the colloidal system, a similar variable in, e.g., volume
concentration is problematic to define even though the experiments require
concentration jumps from one temperature to the next. In order to avoid this
problem in the experiment, a new term, is used to describe aging behavior.
is used to define the
glassy state. However, in the colloidal system, a similar variable in, e.g., volume
concentration is problematic to define even though the experiments require
concentration jumps from one temperature to the next. In order to avoid this
problem in the experiment, a new term, is used to describe aging behavior. ![]() (RTDE = Relaxation
Time Departure from Equilibrium).
(RTDE = Relaxation
Time Departure from Equilibrium).
RESULTS
1. Intrinsic Isotherms
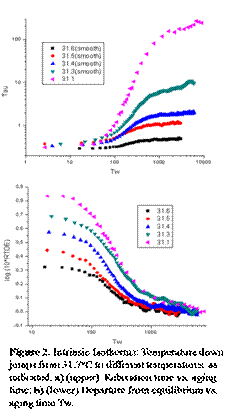 Temperature
was kept at 31.7oC for 3000s to reach equilibrium state before cooling
to 5 different experimental temperatures. The result of Tau vs. Tw is displayed
on Fig.2a All the data except that at 31.1 oC are smoothed by using
moving average function in Origin. Fig.2b shows the relaxation time as a
function of aging time. Similar to glassy polymer, relaxation time at
equilibrium is a strong function of temperature but the time spend to reach
equilibrium state is insensitive to the controlling temperature, which is in
strong contrast to the response observed in molecular glass.
Temperature
was kept at 31.7oC for 3000s to reach equilibrium state before cooling
to 5 different experimental temperatures. The result of Tau vs. Tw is displayed
on Fig.2a All the data except that at 31.1 oC are smoothed by using
moving average function in Origin. Fig.2b shows the relaxation time as a
function of aging time. Similar to glassy polymer, relaxation time at
equilibrium is a strong function of temperature but the time spend to reach
equilibrium state is insensitive to the controlling temperature, which is in
strong contrast to the response observed in molecular glass.
2. Asymmetry of Approach
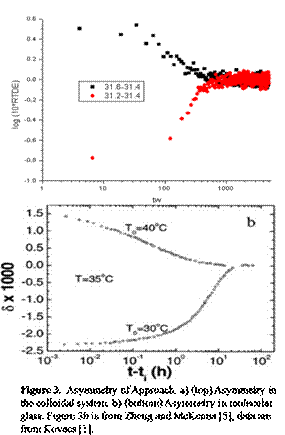 There
are two aspects to the asymmetry of approach experiment, temperature down jump
and temperature up jump. In the down jump, temperature was first kept at 31.6oC
for 4000s and then cooled down to a temperature of 31.4 oC and the
departure from equilibrium monitored as a function of waiting time. In the up
jump experiment, temperature was held at 31.2 oC for 8000s to
equilibrate the sample before heating to 31.4 oC. The colloidal
asymmetry of approach result is compared to that of a molecular glass in Figure
3. We see similar non-linear behavior for the two situations, i.e., the sum
of up-jump and down jump curve does not equal to zero but reaches a minimum
value. For molecular glass, this non-linearity is due to the self-retardation
and self-acceleration because of the long chain configuration of polymer.
However, the reason for non-linearity of colloid is still unknown and under
continuing investigation.
There
are two aspects to the asymmetry of approach experiment, temperature down jump
and temperature up jump. In the down jump, temperature was first kept at 31.6oC
for 4000s and then cooled down to a temperature of 31.4 oC and the
departure from equilibrium monitored as a function of waiting time. In the up
jump experiment, temperature was held at 31.2 oC for 8000s to
equilibrate the sample before heating to 31.4 oC. The colloidal
asymmetry of approach result is compared to that of a molecular glass in Figure
3. We see similar non-linear behavior for the two situations, i.e., the sum
of up-jump and down jump curve does not equal to zero but reaches a minimum
value. For molecular glass, this non-linearity is due to the self-retardation
and self-acceleration because of the long chain configuration of polymer.
However, the reason for non-linearity of colloid is still unknown and under
continuing investigation.
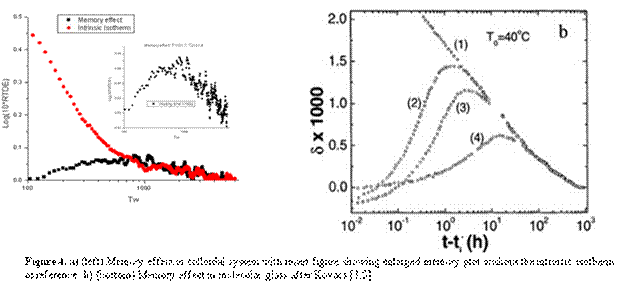
3. Memory effect
In Kovacs's experiments, the volume in any specific experiment was directly measured. However, for the colloid suspension, such a thermodynamic parameter cannot be readily measured and this results in the difficulty of determining the waiting time required to achieve the partial recovery needed to evidence a memory effect in the colloid, should same exist. To solve this problem, several different waiting times were tried to obtain initial values of log(10RTDE) as close to 0 as possible (in the Kovacs experiments he worked to obtain values in the up jump portion of the experiment that started at d=0). Our results show that for the partial recovery temperature of 31.1oC and a final temperature of 31.4oC, the system requires 150s to get close to the relevant initial response at the final temperature. From Fig.4a(insert) , the memory effect peak can be observed clearly although the peak itself is relatively small when compared with the intrinsic isotherm down jump response (Fig.4a). This is different from what Kovacs observed in the molecular glass forming systems polymer (Fig.4b)
CONCLUSIONS
Similar aging signatures are observed in colloidal suspensions to those observed in molecular glasses. However, there appear to be subtle differences between the two types of glass-former. The intrinsic isotherm shows a strong difference in equilibrium relaxation times at different temperatures, but a very weak dependence on the temperature for the time required to reach the equilibrium state; for the asymmetry of approach, similar non-linearity is observed for the colloidal glass but the reason to explain this phenomenon is still unclear as the colloidal system is aging' at constant concentration; finally the memory effects are similar in character for colloid suspension but seem to be of a weaker magnitude. Work is continuing to understand the observed phenomena.
REFERENCE
1. A. J. Kovacs, Forschr. Hochpolym.-Forch., 3, 394 (1963).
2. M. Balauf and Y. Lu, Polymer 48, 1815 (2007).
3. M. Clements, S.R. Pullela, A.F. Mejia, J. Shen, T. Gong, and Z. Cheng, J. Coll. Interface Sci., 317, 96 (2008)
4. V. Viasnoff, F. Lequeux, and D. J. Pine, Rev. Sci. Inst. 73, 2336 (2002).
5. Y. Zheng and G.B. McKenna, Macromolecules, 36, 2387 (2003).
Copyright © American Chemical Society