AmericanChemicalSociety.com
Reports: UNI5 49350-UNI5: Synthesis and Characterization of Molecular Monolayer Directed Nanoscale Catalysts
Kevin M. Metz, PhD, Albion College
The overarching goal of our research through this grant is to produce, study, and understand catalytically active metal nanoparticles on molecular monolayer modified carbon substrates. Specifically, our research hopes to address: 1) the synthesis of uniform metal nanoparticles over large areas; 2) the synthesis of specialized (shaped and/or bimetallic) nanoparticles directly on substrates; and, 3) the catalytic activity of these particles.
In our first year of funding, our research efforts have perused two similar avenues simultaneously. These are the creation and characterization of shaped palladium nanoparticles grown in situ on carbon substrates, and the creation and characterization of gold-palladium bimetallic nanoparticles grown in situ on carbon substrates. Both avenues use molecular monolayers to direct the synthesis of the nanoparticles on the surfaces.
To date, six undergraduate research students have actively participated in this research during the academic year. Three of these students performed research over the summer, one of whom received summer funding through this award. Their efforts have resulted in presentations at the Wayne State University (Detroit, MI) Research Symposium in Chemistry, and two presentations at the 239th National Meeting of the American Chemical Society (San Francisco, CA).
A brief summary of accomplishments to date is included below.
Molecular Monolayer Modification of Carbon Substrates
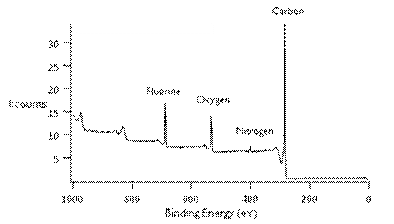
Our approach relies on the presence of molecular monolayers to direct the growth of metal nanoparticles over large areas. Thus, a natural starting point was to insure that we could create the molecular monolayers. Initial studies have used planer vitreous (glassy) carbon, and planer graphite, surfaces functionalized with trifluoroacetic acid protected 10-aminodec-1-ene (TFAAD) using a photochemically mediated reaction using 254 nm light. Funding from the PRF grant was used to purchase a high intensity UV lamp for running these reactions, as well as X-ray photoelectron spectroscopy instrument time at the University of Michigan (Ann Arbor, MI). TFAAD was selected as an initial screening molecule because the presence of the fluorine could differentiate the monolayer from the background signal. Additionally we can quantify the monolayer coverage, again using the fluorine. Figure 1 below shows a representative XPS spectrum for TFAAD on glassy carbon, confirming the presence of fluorine on the surface. Combined with extensive controls samples, these studies have demonstrated our ability to attach TFAAD to carbon surfaces. Based on extensive evidence in the literature, we are comfortable that our techniques are expandable to other molecules that contain directive properties for metal nanoparticle synthesis.
Figure 1. X-Ray
Photoelectron Spectroscopy survey scan spectrum of TFAAD on glassy carbon.
Synthesis of
Palladium and Gold-Palladium Nanoparticles Our goals in synthesizing metals particles are twofold.
First, we want to address fabrication of nanoscale metal nanoparticles over
large areas. Second we want to develop procedures that add significant control
over the deposited metal nanoparticles, creating, for example, bimetallic
and/or shaped nanoparticles. Our initial efforts on fabricating metal
nanoparticles on molecular monolayer modified carbon surfaces have focused on
proof of concept. We have developed a deposition technique based on electroless
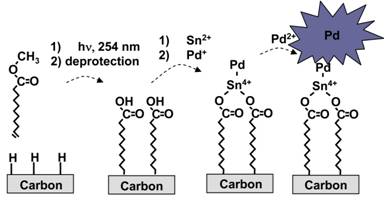
deposition procedures developed for plating metals onto plastics. Figure 2
below outlines the approach we have taken to date. This fabrication procedure
represents a general approach that can be used with multiple metals (e.g.
palladium, platinum, gold, nickel, etc.) and numerous substrates (e.g.,carbon,
silicon, etc.) to develop metal nanoparticles with different catalytic
properties.
Figure 2. Schematic
representation of our approach for depositing PdNPs on molecular monolayers.
To date we have been
successful using this technique to deposit uniformly sized palladium
nanoparticles (PdNPs) and uniformly sized gold nanoparticles (AuNPs) on glassy
carbon substrates. Progress has been slower than anticipated, however, due to
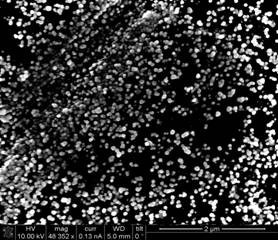
limitations to instrument access. Figure 3 below shows a scanning electron
microscope (SEM) image of a representative glassy carbon substrate covered in
PdNPs. At current we are capable of
depositing uniform PdNPs with diameters in the 25 ± 10 nm range and uniform
AuNPs in the 10-20 nm diameter range over the entire area of our substrates.
Funds from the PRF grant have been used to purchase SEM time at the University
of Michigan and at Michigan State University.
Figure 3. Scanning
electron microscope image of uniformly sized PdNPs on a molecular monolayer
modified glassy carbon substrate.
It is anticipated that
for the remainder of the funding period for this grant we will focus on
improving our nanoscale metal deposition capabilities focusing on controlling
shape and fabricating bimetallic NPs, while simultaneously beginning to
analysis the catalytic properties of our deposited metals.
Copyright © American Chemical Society