AmericanChemicalSociety.com
Reports: UNI6 49343-UNI6: Room Temperature Chirped-Pulse Fourier Transform Microwave Spectroscopy for the Study of Radical Reaction Dynamics
Steven T. Shipman, PhD, New College of Florida
The goal of this project is to use chirped-pulse Fourier transform microwave (CP-FTMW) spectroscopy from 8.7 – 18.5 GHz as a probe of gas phase chemical reactions occurring at low pressures (1 – 100 mTorr) and at room temperature. The original proposal discussed reactions between small unsaturated hydrocarbons (such as propene) and hydroxyl radicals, created from hydrogen peroxide by pulses of UV light.
These measurements utilize the core of a CP-FTMW spectrometer that was constructed at New College of Florida via start-up funding. Funding from ACS-PRF has provided a UV light source, a 2-meter long “sample cell” of WRD-750 waveguide, and associated optical windows and vacuum components associated with transmitting light and molecules in the gas phase into and out of the sample cell. To date, this award has also supported two undergraduates for 10 weeks of summer research. Benjamin (Ben) Kriegel '11 worked in the lab during the summer of 2009, and Sophie Lang '13 worked in the lab during the summer of 2010. Ben is currently applying to chemistry graduate schools, and Sophie intends to graduate with a B.A. in Chemistry in the spring of 2013.
Work began on this project during the summer of 2009. Both the microwave and the optical components
had long lead times and so a majority of the purchased equipment did not arrive
until mid-August, with the last pieces arriving in October. Despite these delays, we made progress during
the summer by using room-temperature CP-FTMW spectroscopy to perform initial
characterization of the unsaturated hydrocarbon reactants as well as the
closed-shell forms of some of the potential products. During that summer, Ben Kriegel
collected and performed preliminary analysis on the room-temperature rotational
spectra of seven molecules – propene, 1-butene, isoprene, 1-propanol,
2-propanol, 1-butanol, and 2-butanol. As
the During the summer of 2010, Sophie Lang worked in the lab
both to implement schemes for microwave-microwave double resonance (to aid in
assignment of the complex spectra we expect to find) and to incorporate the UV
flash lamp into the experimental setup.
Her double resonance work, which effectively “tags” spectral features
that share a quantum state with a pumped feature, was successful (Figure 2). Reaction initiation with the flash lamp was
less successful. Her initial plan was to
monitor depletion of the propene signal from a propene / hydrogen peroxide gas
mixture as a function of UV exposure time.
Early attempts at these measurements were unsuccessful due to both the
small propene microwave signal, resulting from its low dipole moment of ~ 0.3
D, and the relatively small number of photons produced by the UV flash
lamp.
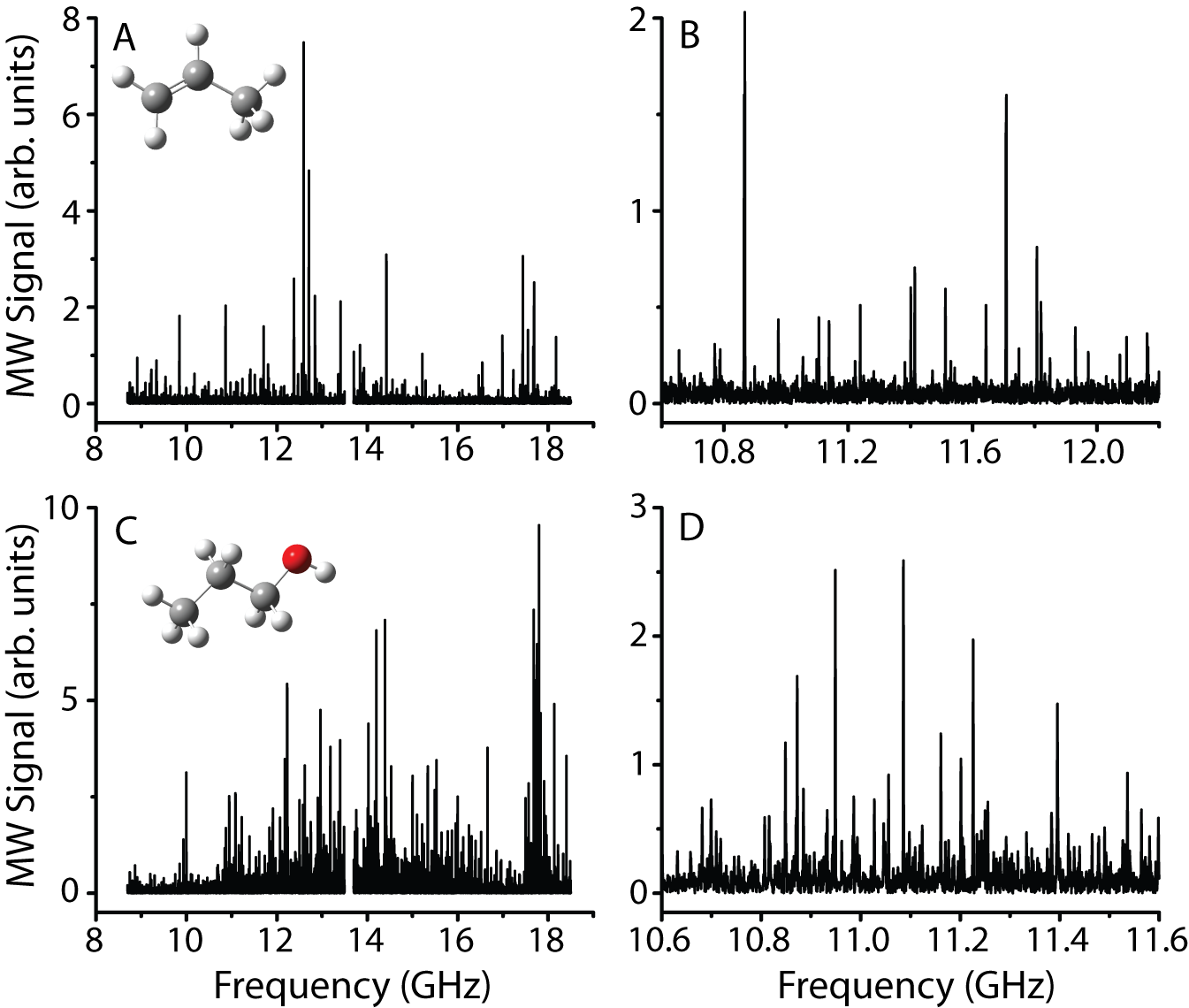
Figure
1. Panels A and B (expanded view of A) show the
room-temperature microwave spectrum of propene, and panels C and D (expanded
view of C) show the room-temperature microwave spectrum of 1-propanol. Each spectrum represents roughly 16 hours of
data collection. Figure
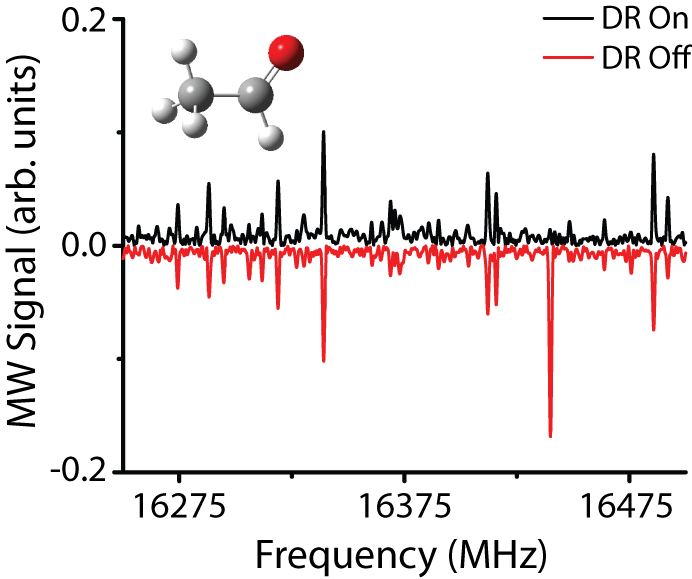
2. A microwave-microwave
double resonance measurement on acetaldehyde. The positive-going spectrum is collected in
the presence of the double-resonance pulse, and the negative-going spectrum is
collected in the absence of the double-resonance pulse. The double-resonance pulse is tuned to the
acetaldehyde nt = 1, 514-
– 515+ transition at 14548.83 MHz, and has drastically modulated the
intensity of the nt = 1, 423-
– 514- transition at 16439.93 MHz without significantly affecting
other features in the spectrum. Only
transitions that share a common quantum state with the pumped transition (the nt = 1, 514-
state in this case) show an intensity modulation when the double-resonance
pulse is applied. To improve the signal-to-noise ratio of the propene
microwave signal, we changed the polarizing pulse parameters and constructed a
modified receiver circuit which allowed us to strike a balance between
detection bandwidth and repetition rate.
Specifically, by reducing the polarizing pulse bandwidth from 5 GHz to
50 MHz and by judiciously choosing frequency sources and microwave filters to
reduce the detection bandwidth from 5 to 1.25 GHz, we have been able to
simultaneously quadruple the repetition rate and boost the propene signal by a
factor of 10. Combined, these
modifications lead to a 20-fold enhancement of the signal-to-noise ratio of the
propene transition in equal measurement time (Figure 3).
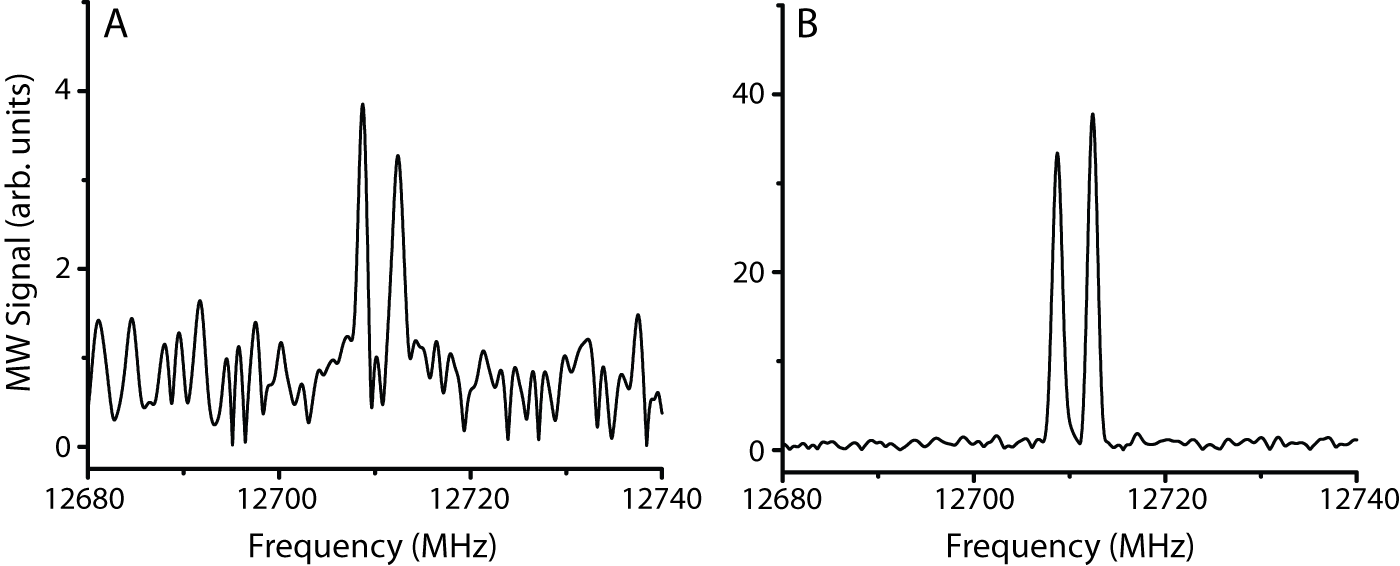
Figure
3. Propene spectra acquired with a 5 GHz
polarizing pulse, FID digitized at 10 GS/s (panel A) and with a 50 MHz
polarizing pulse, FID digitized at 2.5 GS/s (panel B). Both spectra represent 20 minutes of
averaging; these two spectra represent extremes in the tradeoffs possible
between spectral coverage (5 GHz vs. 50 MHz) and overall signal-to-noise ratio. To address the light intensity issue, we will be using a 266
nm, Q-switched laser as our UV light source, courtesy of a colleague in
physics, Prof. Mariana Sendova. Over the next year, we plan to use this
improved light source to make initial measurements on propene and extend them
to 1-butene and isoprene. Because
hydrogen peroxide has very few rotational transitions in the frequency range of
the spectrometer, it is difficult to follow its destruction with UV
irradiation. As such, in the coming year
we also plan to look into slightly larger radical precursors that both
dissociate under UV irradiation and have transitions that are easily monitored
via their rotational spectra.
Copyright © American Chemical Society