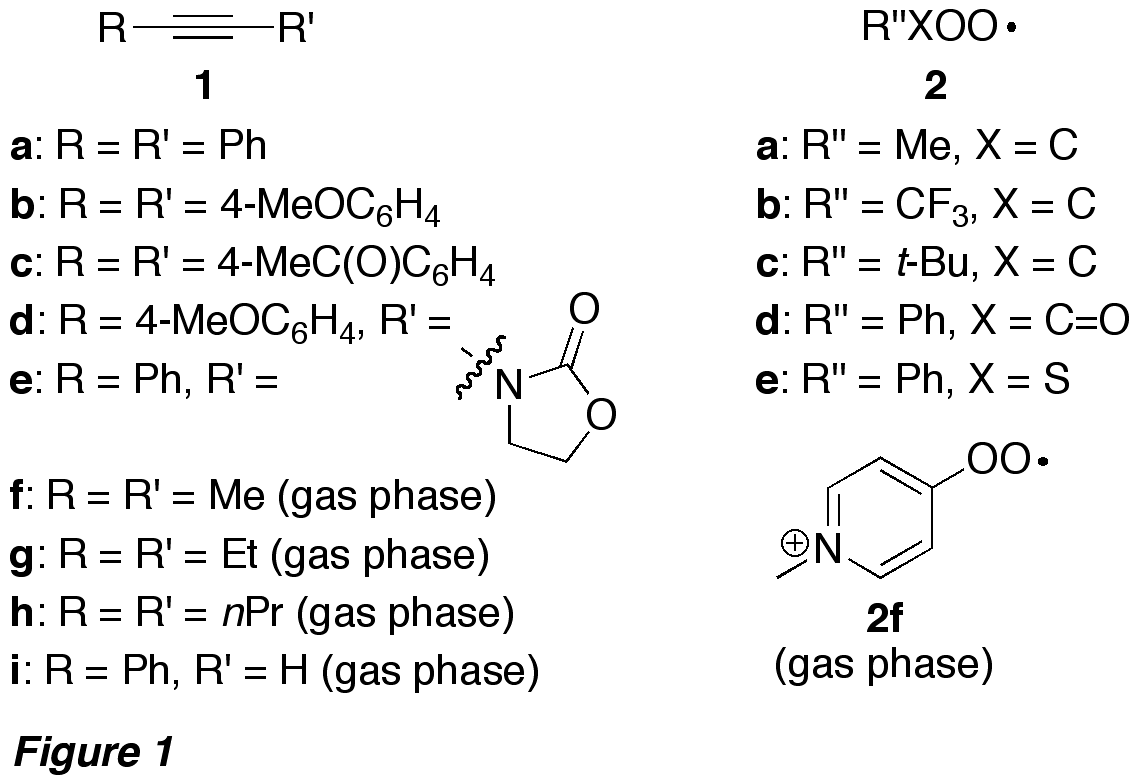AmericanChemicalSociety.com
Reports: AC4 48217-AC4: Development of New Pathways for the Oxidative Transformation of Alkynes into Highly Reactive Carbonyl Compounds
Uta Wille, The University of Melbourne
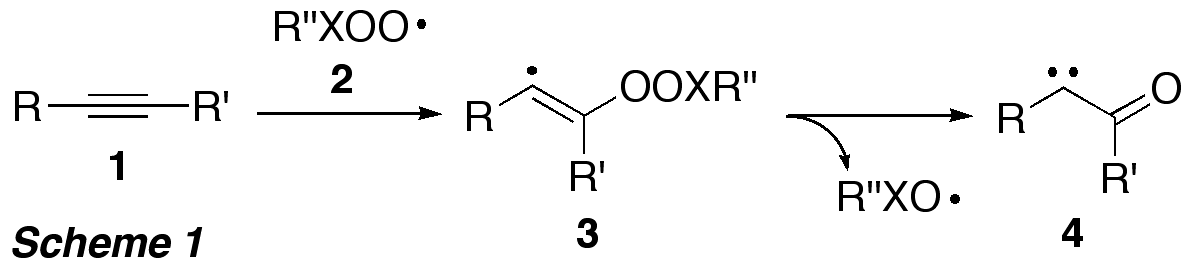
The development of novel oxidation procedures that use the most abundant (and cheapest) oxidant, molecular oxygen, under non-toxic conditions is a major goal in synthetic chemistry. This project used a combination of experimental (solution and gas phase) and computational methods to explore, whether peroxyl radicals R''XOO (2, X = S, C) can be used to transform alkynes into carbonyl compounds. According to computational predictions, addition of R''XOO to the alkyne triple bonds in 1 should give vinyl radical 3 (Scheme 1), which, should undergo homolytic gamma-fragmentation of the weak peroxyl bond in 3 to form alpha-oxo carbene 4, potentially via an intermediate oxirene (not shown).
Since R''XOO are easily accessible
through reaction of the respective C- or S-centred radical R''X with oxygen,
this sequence would enable generation of highly reactive and synthetically very
useful alpha-oxo carbenes in a single step from simple alkynes.
In Figure 1 are shown the alkynes
and peroxyl radicals studied. For the gas phase experiments in the mass
spectrometer, the charged peroxyl radical 2f was used. For the
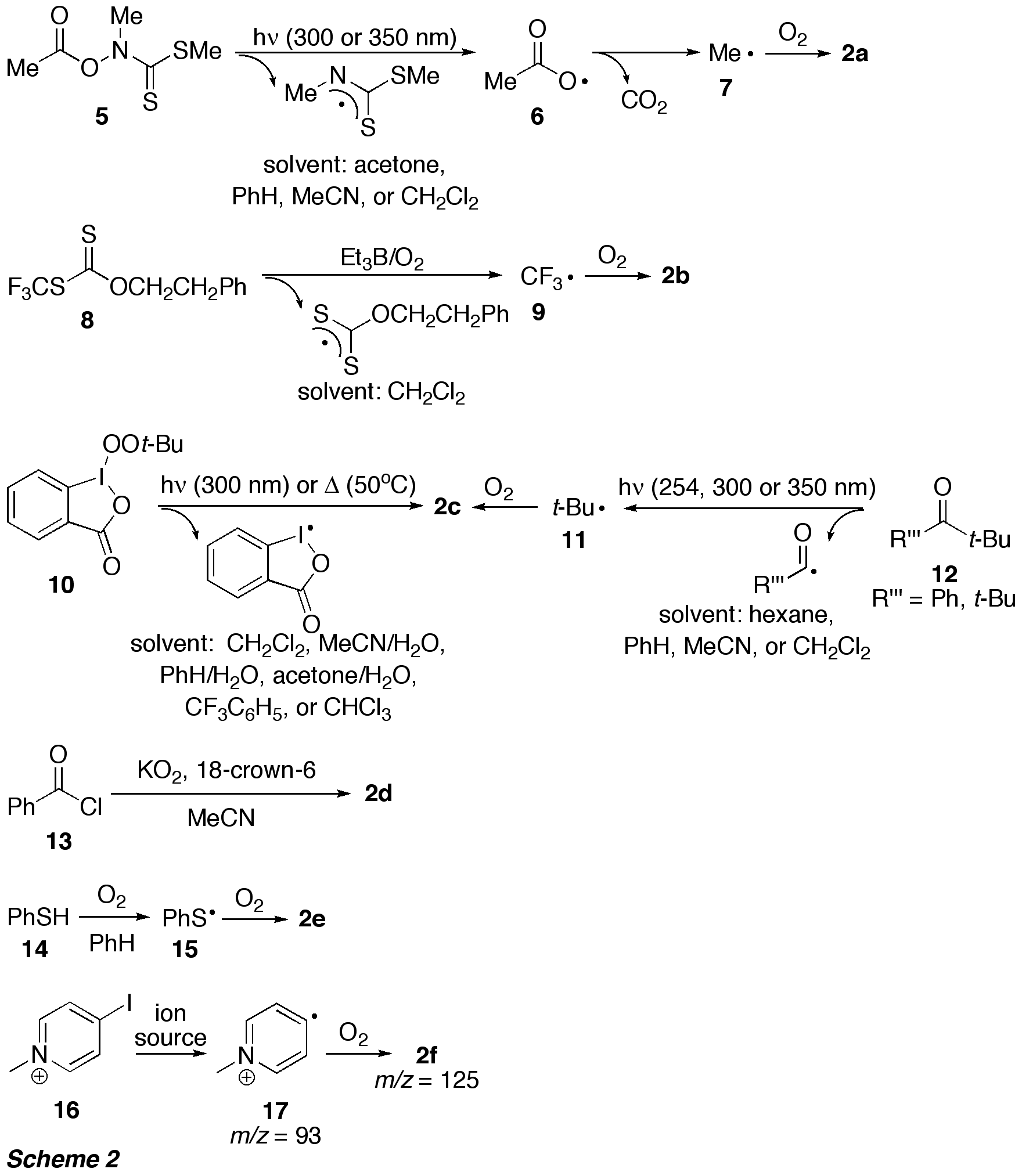
experiments in solution, peroxyl radicals 2a-e were generated in the presence of the alkyne by both photochemical and
thermal methods (Scheme 2). For the gas phase experiments performed in a mass
spectrometer, the charged peroxyl radical 2f was generated by reaction of aryl radical 17 with O2 in the ion source, and
subsequently mass selected into the ion trap, where it reacted with the alkyne.
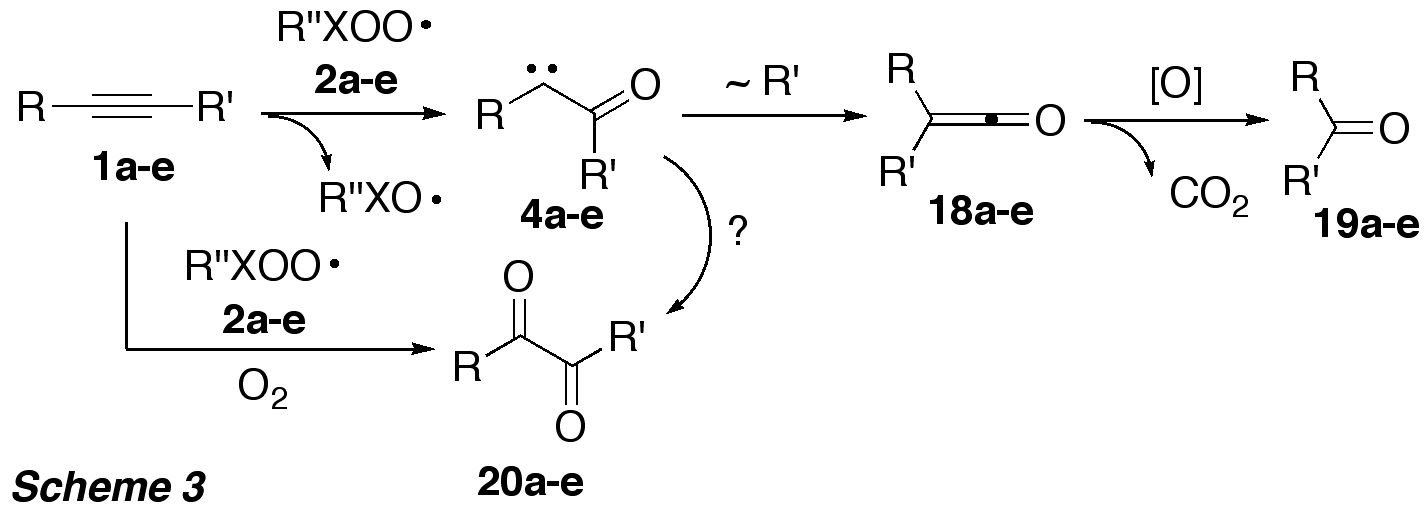
Computations predicted that
intermediately formed alpha-oxo carbenes 4a-d should undergo Wolff-Rearrangement, and the resulting ketenes 18a-d could be further oxidized (by peroxyl radicals) to
the corresponding ketones 19a-d
(Scheme 3). Their formation would provide evidence for the success of the
reaction sequence.
Interestingly, only in the reaction
of diphenylacetylene (1a) with peroxyl
radical 2c formation of
benzophenone (19a) was observed,
however with only very low yield (<10%). The rate of the addition of the
electrophilic peroxyl radicals was strongly influenced by the electron density
at the alkyne pi system. In the reactions of 2c with alkynes 1a,b,d the corresponding 1,2-diketones 20a,b,d were unexpectedly formed. This discovery is
highly relevant from a synthetic point of view, since this reaction may be
considered as a very mild alternative to the existing oxidation procedures of
alkynes to 1,2-diketones, which generally require harsh conditions and toxic
heavy metal compounds. Most importantly, since peroxyl radicals are commonly
produced by reaction of C- or S-centred radicals with oxygen, this methodology
is can be regarded as an environmentally benign, radical
mediated metal-free activation of oxygen.
It appeared that presence of oxygen
was crucial for the transformation 1 ->
20 by peroxyl radicals: When
alkyne 1a was reacted with t-BuOO
(2c), which was generated by thermal
decomposition of the hypervalent iodocompound 10 in the absence of oxygen (see Scheme 2), diketone 20a was not formed and no reaction occurred. For the
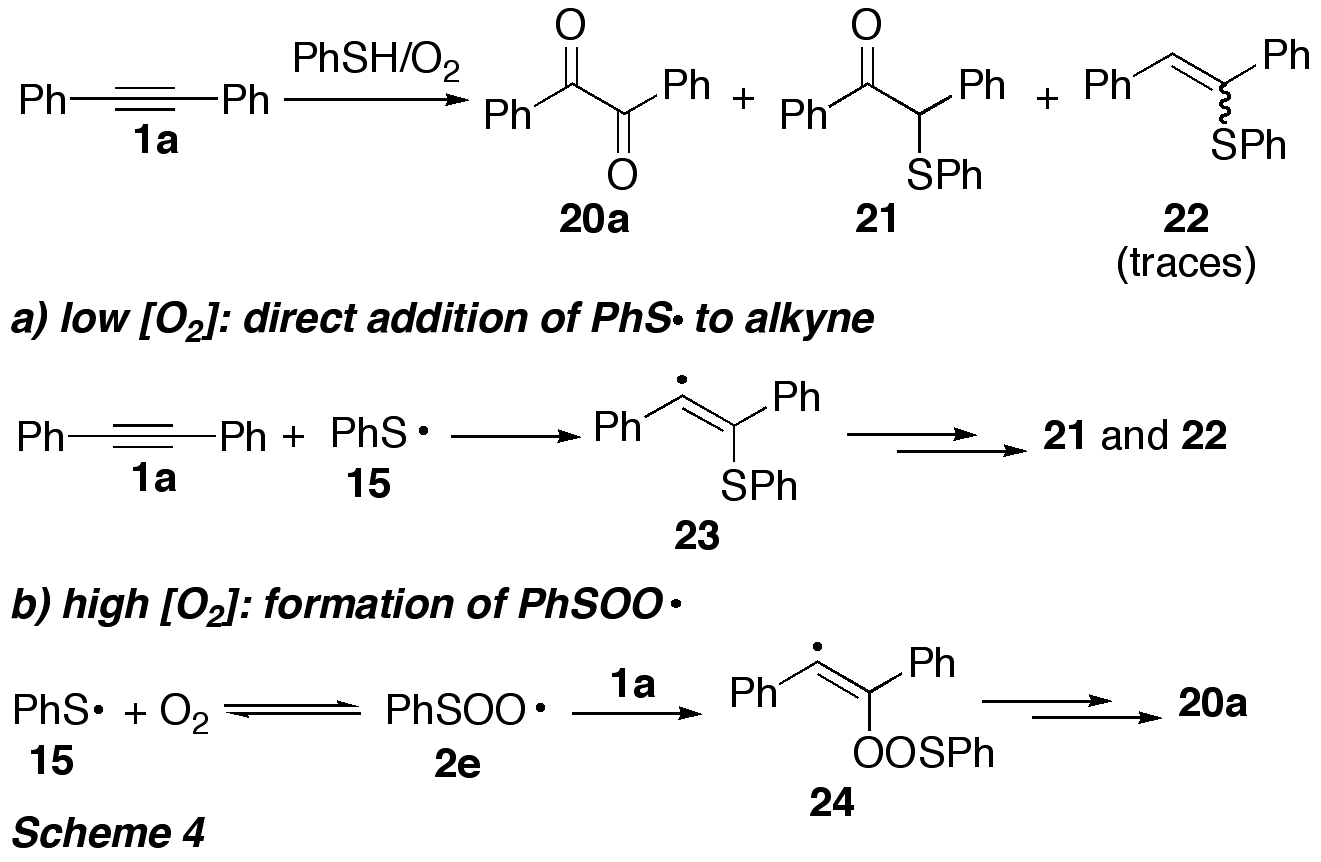
reaction involving S-radicals the influence of oxygen was studied in detail,
since the reaction pathway depended on the oxygen concentration (Scheme 4). At low [O2], direct
addition of the S-radical 15 to the
alkyne occurred, resulting in formation of the thioethers 21 and 22.
When [O2] was increased, the yield of diketone 20a increased, at the expense of 21 and 22.
Under these conditions, S-radicals 15 were trapped by oxygen to give peroxyl radicals 2e, which undergo addition to the alkyne triple bond.
The sequence shown in Scheme 4 is the first example for a synthetic application
of thiylperoxyl radicals.
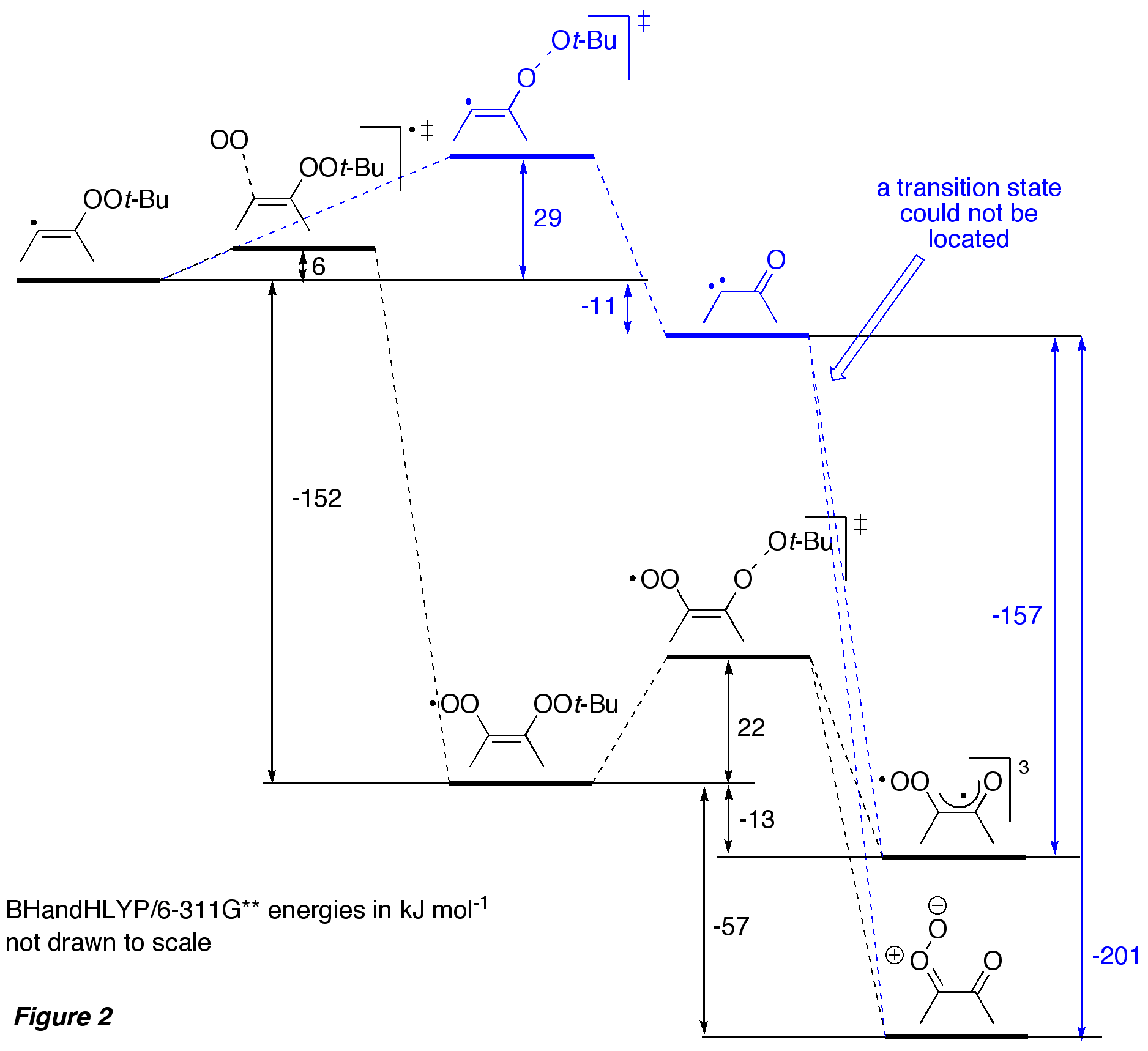
The mechanism for formation of
diketones 20 has been explored with DFT
computations for an exemplary model system, and the calculated potential energy
surface is shown in Figure 2. It appears that trapping of the
initially formed vinyl radical by oxygen is more favourable than
gamma-fragmentation of the O–O bond to give carbene, which is
subsequently trapped by oxygen. The prediction that under our experimental
conditions a free carbene is not formed, was supported by additional
independent experimental studies (not shown). In order to gain more fundamental
experimental insight into the mechanism of peroxyl radical additions to
alkynes, we have performed mass spectrometric studies to identify the primary
products of these reactions in the gas phase. The reactions were performed with
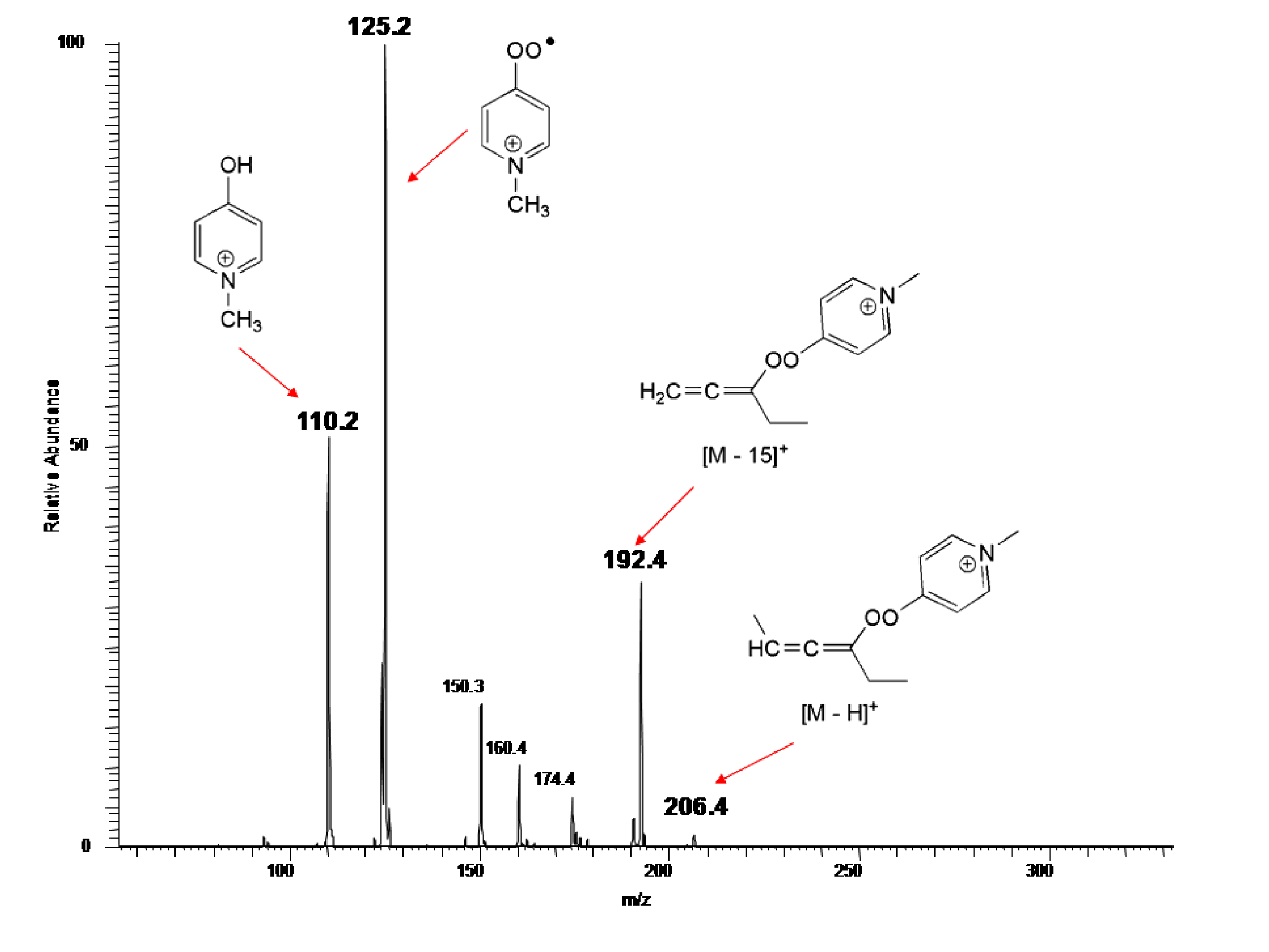
alkynes 1f-i and the charged peroxyl radical 2f. In Figure 3 is exemplary shown the mass spectrum
of the reaction of 2f with an
excess of alkyne 1f after 1 min
reaction time. Although these reactions are not fast, in general, addition of
the peroxyl radical to the pi system is the major pathway, whereas propargylic
hydrogen abstraction is only occurring to a minor extent. According to the
data, the initially formed vinyl radical undergoes a number of fragmentation
reactions, which have not been fully identified so far. However, the signal at
m/z 110 supports the predicted fragmentation 3 ¨ 4 (see Scheme 1), which releases an alkoxyl radical that is rapidly
reduced to the respective alcohol through hydrogen transfer. Figure
3 Apart from these exciting
"snapshots" of the alkyne – peroxyl radical reactions, these gas phase
studies have provided rate data for the formation of peroxyl radicals (e.g. 17 ¨ 2f) and their addition to alkynes. This is an important outcome, since
rate data for intermolecular radical additions, specifically of O-radicals, to
alkynes are surprisingly rare. Although the financial support by
the PRF is now expired, this project is not finished by any means. Our research
during the 2 year funding period has opened up exciting new directions for our
research, specifically in the area of fundamental radical kinetics and transient
spectroscopy. In the future, we will further explore this novel metal-free
activation of oxygen for the oxidative transformation of pi systems into
carbonyl compounds. The involved PhD will soon submit his thesis, and he has
already presented the results on various national and international conferences
through oral and poster presentations. It is intended that at least two
research papers on this work will be submitted for publications in the next
months.
Copyright © American Chemical Society