AmericanChemicalSociety.com
Reports: DNI7 49218-DNI7: Assembly of Organometallics in Polymeric Templates for Metal Coated Mesoporous Materials
Bryan D. Vogt, Arizona State University
This work focuses on understanding the assembly of surfactants, carbonizable precursors and organometallic compounds to fabricate nanoporous composite materials that have potential use in catalysis and energy storage (batteries/supercapacitors). Several different carbonizable precursor routes were explored: (1) in-situ reactive where phenol/resorcinol/phloroglucinol and formaldehydye are polymerized during self-assembly, (2) post polymerization where phenol/resorcinol/phloroglucinol and an acid are initially added to the precursor solution and the aromatic alcohols are polymerized using formaldehyde vapor, and (3) non-reactive where an oligomer of phenol-formaldehyde (PF) is added as the carbonizable precursor. Route (1) results in phase separation of the templating solution and a very limited shelf life for the solution prior to solidification. Route (2) is limited to thin films as diffusion of the formaldehyde is required for polymerization. One issue with this approach is that the aromatic alcohol can crystallize partially during annealing and polymerization with formaldehyde; this crystallization appears to lead to phase separation and formation of large pores. An example of this method is shown below in Figure 1.
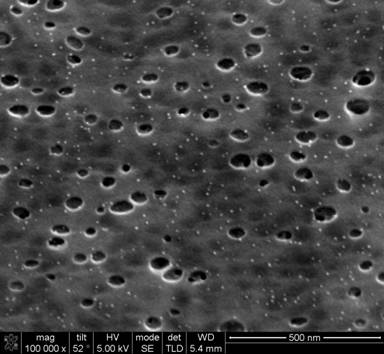
Figure 1. SEM image of porous carbon-vanadium oxide film formed using route (2). Bright dots are vanadium oxide nanoparticles formed in-situ and dark regions are the pores |
The organometallic compound, vanadium acac in this case, is decomposed during carbonization of the resorcinol-formaldehyde (RF) resin and removal of the surfactant. This yields 5-10 nm nanoparticles of vanadium oxide (oxygen is provided from the RF resin). Although this synthesis route does yield porous carbons decorated with nanoparticles, it is limited to thin films and requires multiple steps in the synthesis.
To overcome these issues, another
method based upon the co-assembly of PF oligomer,
surfactant and organometallic has been explored in
attempts to form ordered mesoporous nanocomposites. This
route is attractive from a processing prospective (single step to form powders
or thin films) and the solutions are stable unlike many sol-gel materials. This later property enables the same mesostructure to be reproducibly fabricated from the same
solution; we have tested 1 month old solutions with no change in
morphology. Figure 2 illustrates an
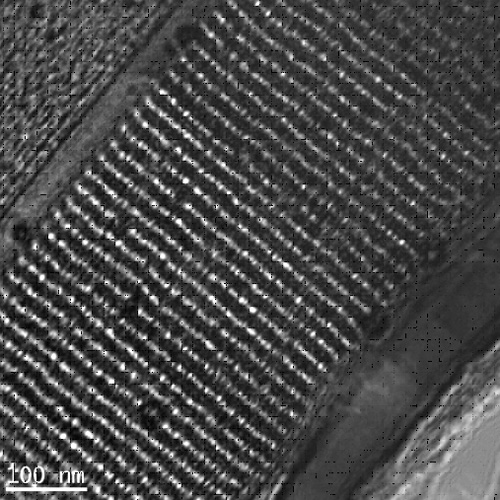
ordered mesoporous carbon composite. In this case, the film contains 1.7 wt %
Figure 2. TEM image of porous carbon-cobalt oxide film formed using route (3). In this case, a highly ordered mesostructure is formed. The film contains 1.7 wt% Co Figure 3 illustrates the low
diffraction of the mesoporous materials formed using Co(acac) as the organometallic.
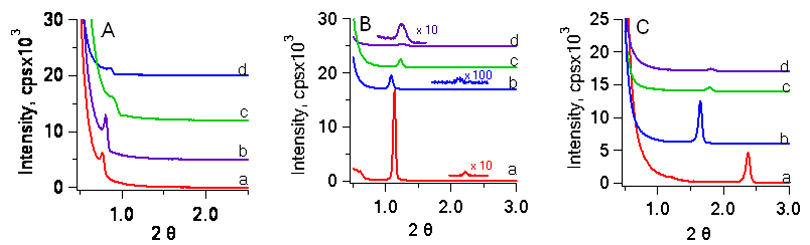
Initially the mesostructure formed from the
assembly of resol, surfactant and Co(acac) does not appear to be significantly impacted by the
concentration of Co(acac) for the as-made
materials. The surfactant can be fully
removed at 350 C, but the resol is not converted to
carbon at this temperature. As can be
seen in Figure 3B, the location of the diffraction peaks is shifted to higher
angles by pyrolysis at 350 C, but still there is no
dependence on the Co(acac)
concentration. Interestingly,
carbonization at 800 C leads to significant differences in the location of the
primary diffraction peak. There is
significantly less contraction in mesoporous
materials that contain some Co(acac);
this suggests that some Co is incorporated into the pore walls in addition to
the nanoparticles.
We have found similar results when adding silica (via TEOS) to these mesoporous carbons.
Figure 3. XRD profiles of (A) as-made materials, (B) mesoporous polymer composites and (C) mesoporous carbon nanocomposites from solutions containing (a) 0 wt %, (b) 10 wt %, (c) 20 wt % and (d) 33 wt % Co(acac). The pore size distribution of
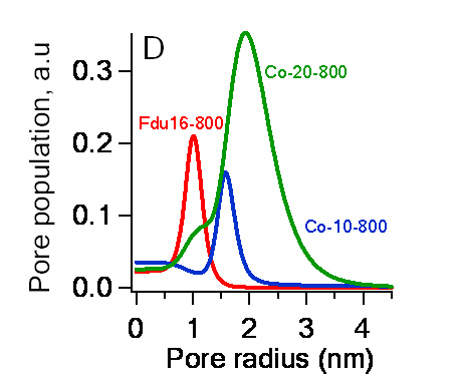
these materials also provides insight into the role of the Co(acac) on the pore morphology. As can be seen in Figure 4, the average pore
size shifts to larger radii with the addition of Co(acac). This result
is consistent with XRD that shows a decrease in the contraction extent with
added Co(acac).
Figure 4. Pore size distributions for mesoporous carbon films containing 0 wt % Co (FDU-16-800), 1.7 wt % Co (Co-10-800) and 3.3 wt % Co (Co-20-800). We have found similar results
using V(acac) as a
precursor. With the morphology of these
materials now understood, we are now focusing on the electrochemical properties
of these materials for supercapacitors. Additionally, the catalytic properties of the
Co3O4 containing mesoporous
carbons for the oxygen reduction reaction (ORR) will be investigated. Co3O4 containing porous
carbons have recently been shown to be effective for ORR, but it is strongly
dependent upon the morphology. If
successful, these materials could be utilized as electrodes in fuel cells for
ORR.
This award has enabled my
research to advance into a new application area of electrochemical storage. This seed funding has been instrumental in
obtaining preliminary data for DOE and NSF proposals (still pending) that would
not have been possible. This award has
also provided the final year of support for my first Ph.D. student
(graduated). She is currently employed
at the Arizona Power Services (APS), working on applied research towards
improving energy efficiency. A second
Ph.D. student is now supported on this award and has begun to examine the
electrochemical performance of the synthesized materials.
Copyright © American Chemical Society